Abstract
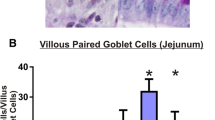
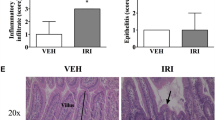
Irinotecan–induced mucositis is a major oncological problem. Goblet cells secrete mucus, protecting the intestinal mucosa, with secretion altered during mucositis. The enteric nervous system is involved in regulating gut motility and secretion. The aim of this study was to determine whether enteric neural cells and goblet cells are altered following irinotecan treatment. Tumour-bearing Dark Agouti rats were administered a single dose of 175 mg/kg of irinotecan intraperitoneally and 0.01 mg/kg atropine subcutaneously. Experimental and untreated control rats were killed at times 6, 24, 48, 72, 96 and 120 h after treatment. Jejunum and colon samples were formalin fixed. Haematoxylin and eosin staining, Alcian Blue-PAS staining, and immunohistochemistry with S-100 antibody (neural cell marker) were carried out. Statistical analyses were carried out using Kruskal-Wallis test with Dunns post test, Mann Whitney U test and nonlinear regression. Total goblet cells decreased at 72 h compared with controls in the colon (p < 0.05). The percentage of cavitated goblet cells decreased compared to all other time points at 120 h in the colon. The number of S-100 positive cells in the submucosal plexus decreased in the colon (p = 0.0046) and in the myenteric plexus of the jejunum and colon (p = 0.0058 and p = 0.0022, respectively), when comparing treated with control. Enteric ganglia in the myenteric plexus of the jejunum decreased at 24 h and 96 h. Irinotecan-induced mucositis is associated with increases in mucus secretion, and enteric neural cell change. These changes may contribute to the pathophysiology of mucositis through the dysregulation of neural signalling.





Similar content being viewed by others
References
Keefe DM, Brealey J, Goland GJ, Cummins AG (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47(5):632–637
Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, Sage RE (1997) Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 92(4):385–389
Pico JL, Avila-Garavito A, Naccache P (1998) Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist 3(6):446–451
Keefe DM (2004) Gastrointestinal mucositis: a new biological model. Support Care Cancer 12(1):6–9. https://doi.org/10.1007/s00520-003-0550-9
Sonis ST, Costa JW Jr, Evitts SM, Lindquist LE, Nicolson M (1992) Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive cancer chemotherapy. Oral Surg Oral Med Oral Pathol 74(6):749–755
Savarese DM, Hsieh C, Stewart FM (1997) Clinical impact of chemotherapy dose escalation in patients with hematologic malignancies and solid tumors. J Clin Oncol 15(8):2981–2995
Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98(7):1531–1539. https://doi.org/10.1002/cncr.11671
Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68(4):1110–1120. https://doi.org/10.1016/j.ijrobp.2007.01.053
Gibson RJ, Keefe DM, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ, Yazbeck R, Elad S, Bowen JM (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer 21(1):313–326. https://doi.org/10.1007/s00520-012-1644-z
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10):1453–1461. https://doi.org/10.1002/cncr.28592
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109(5):820–831. https://doi.org/10.1002/cncr.22484
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025. https://doi.org/10.1002/cncr.20162
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(9 Suppl):2026–2046. https://doi.org/10.1002/cncr.20163
Saltz LB (2003) Understanding and managing chemotherapy-induced diarrhea. J Support Oncol 1(1):35–46; discussion 38-41, 45-36
Wadler S, Benson AB 3rd, Engelking C, Catalano R, Field M, Kornblau SM, Mitchell E, Rubin J, Trotta P, Vokes E (1998) Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol 16(9):3169–3178
Al-Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, Gibson RJ, Logan RM, Nair RG, Stringer AM, Yazbeck R, Elad S, Lalla RV (2013) Emerging evidence on the pathobiology of mucositis. Support Care Cancer 21(7):2075–2083. https://doi.org/10.1007/s00520-013-1810-y
de Koning BA, Sluis M, Lindenbergh-Kortleve DJ, Velcich A, Pieters R, Buller HA, Einerhand AW, Renes IB (2007) Methotrexate-induced mucositis in mucin 2-deficient mice. J Cell Physiol 210(1):144–152. https://doi.org/10.1002/jcp.20822
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Laurence J, Keefe DM (2009) Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol 64(1):123–132. https://doi.org/10.1007/s00280-008-0855-y
Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM (2009) Gastrointestinal microflora and mucins may play a critical role in the development of 5-fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood) 234(4):430–441. https://doi.org/10.3181/0810-rm-301
Saegusa Y, Ichikawa T, Iwai T, Goso Y, Okayasu I, Ikezawa T, Shikama N, Saigenji K, Ishihara K (2008) Changes in the mucus barrier of the rat during 5-fluorouracil-induced gastrointestinal mucositis. Scand J Gastroenterol 43(1):59–65
Verburg M, Renes IB, Meijer HP, Taminiau JA, Buller HA, Einerhand AW, Dekker J (2000) Selective sparing of goblet cells and paneth cells in the intestine of methotrexate-treated rats. Am J Physiol Gastrointest Liver Physiol 279(5):G1037–G1047
Thorpe D, Stringer A, Butler R (2013) Chemotherapy-induced mucositis: the role of mucin secretion and regulation, and the enteric nervous system. Neurotoxicology 38:101–105. https://doi.org/10.1016/j.neuro.2013.06.007
Wood JD (2011) Enteric nervous system: the brain-in-the-gut. In: Integrated systems physiology: from molecule to function to disease, vol 26. Morgan & Claypool Life Sciences, Ohio
Branka JE, Vallette G, Jarry A, Laboisse CL (1997) Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem J 323(Pt 2):521–524
Plaisancie P, Barcelo A, Moro F, Claustre J, Chayvialle JA, Cuber JC (1998) Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Phys 275(5 Pt 1):G1073–G1084
Gibson RJ, Keefe DM, Thompson FM, Clarke JM, Goland GJ, Cummins AG (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47(12):2751–2757
Gibson RJ, Keefe DM, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG (2002) The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 50(1):53–58. https://doi.org/10.1007/s00280-002-0460-4
Bowen JM, Gibson RJ, Cummins AG, Tyskin A, Keefe DM (2007) Irinotecan changes gene expression in the small intestine of the rat with breast cancer. Cancer Chemother Pharmacol 59(3):337–348. https://doi.org/10.1007/s00280-006-0275-9
Gibson RJ, Bowen JM, Keefe DM (2005) Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer 116(3):464–470. https://doi.org/10.1002/ijc.21082
Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancie P (2000) Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46(2):218–224
Ilg EC, Schafer BW, Heizmann CW (1996) Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer 68(3):325–332. https://doi.org/10.1002/(sici)1097-0215(19961104)68:3<325::aid-ijc10>3.0.co;2-7
Bowen JM, Gibson RJ, Keefe DM, Cummins AG (2005) Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37(1):56–62
Cohen J (2013) Statistical power analysis for the behavioral sciences. Taylor & Francis, New York
Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DM (2008) Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62(1):33–41. https://doi.org/10.1007/s00280-007-0570-0
Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18(9):1095–1100
Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh AS, Al-Dasooqi N, Keefe DM (2009) Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90(5):489–499. https://doi.org/10.1111/j.1365-2613.2009.00671.x
Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T (1996) Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56(16):3752–3757
Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T (1998) Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol 42(4):280–286
Takasuna K, Hagiwara T, Watanabe K, Onose S, Yoshida S, Kumazawa E, Nagai E, Kamataki T (2006) Optimal antidiarrhea treatment for antitumor agent irinotecan hydrochloride (CPT-11)-induced delayed diarrhea. Cancer Chemother Pharmacol 58(4):494–503. https://doi.org/10.1007/s00280-006-0187-8
Cao XJ, Wang WM (2009) Histology and mucin histochemistry of the digestive tract of yellow catfish, Pelteobagrus fulvidraco. Anat Histol Embryol 38(4):254–261. https://doi.org/10.1111/j.1439-0264.2009.00932.x
Truter D, Strijdom H, Everson F, Kotze SH (2016) Mucin secreting cells in the stomach and colon are altered by combination antiretroviral treatment in an obese rat model. Acta Histochem 119:122–128. https://doi.org/10.1016/j.acthis.2016.11.014
Van Herreweghen F, Van den Abbeele P, De Mulder T, De Weirdt R, Geirnaert A, Hernandez-Sanabria E, Vilchez-Vargas R, Jauregui R, Pieper DH, Belzer C, De Vos WM, Van de Wiele T (2016) In vitro colonisation of the distal colon by Akkermansia muciniphila is largely mucin and pH dependent. Benefic Microbes 8:1–16. https://doi.org/10.3920/bm2016.0013
Laburthe M, Augeron C, Rouyer-Fessard C, Roumagnac I, Maoret JJ, Grasset E, Laboisse C (1989) Functional VIP receptors in the human mucus-secreting colonic epithelial cell line CL.16E. Am J Phys 256(3 Pt 1):G443–G450
Gutierrez MM, Alluisetti GB, Olabe JA, Amorebieta VT (2008) Nitrosation of N-methylhydroxylamine by nitroprusside. A kinetic and mechanistic study. Dalton Trans 37:5025–5030. https://doi.org/10.1039/b805329d
Phillips TE, Stanley CM, Wilson J (1993) The effect of 16,16-dimethyl prostaglandin E2 on proliferation of an intestinal goblet cell line and its synthesis and secretion of mucin glycoproteins. Prostaglandins Leukot Essent Fat Acids 48(6):423–428
Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM (2008) Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 7(7):1139–1145
Specian RD, Neutra MR (1980) Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol 85(3):626–640
Specian RD, Neutra MR (1982) Regulation of intestinal goblet cell secretion. Am J Phys 242(4):G370–G379
Lake AM, Bloch KJ, Sinclair KJ, Walker WA (1980) Anaphylactic release of intestinal goblet cell mucus. Immunology 39(2):173–178
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73(6):1131s–1141s
Furness JB, Kunze WA, Clerc N (1999) Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Phys 277(5 Pt 1):G922–G928
Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9(5):286–294. https://doi.org/10.1038/nrgastro.2012.32
McCool DJ, Marcon MA, Forstner JF, Forstner GG (1990) The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J 267(2):491–500
Turner JE, Stockinger B, Helmby H (2013) IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 9(10):e1003698. https://doi.org/10.1371/journal.ppat.1003698
Sultani M, Stringer AM, Bowen JM, Gibson RJ (2012) Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemother Res Pract 2012:490804–490811. https://doi.org/10.1155/2012/490804
Funding
This study was funded by NHMRC funding (1016696).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Dr. Daniel Thorpe declares that he has no conflict of interest, Ms. Masooma Sultani declares that she has no conflict of interest, Professor Ross Butler declares he has no conflict of interest, Dr. Barbara Vanhoecke declares she has no conflict of interest, and Dr. Andrea Stringer declares that she has no conflict of interest. Furthermore, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and all procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thorpe, D., Butler, R., Sultani, M. et al. Irinotecan-Induced Mucositis Is Associated with Goblet Cell Dysregulation and Neural Cell Damage in a Tumour Bearing DA Rat Model. Pathol. Oncol. Res. 26, 955–965 (2020). https://doi.org/10.1007/s12253-019-00644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00644-x