Abstract
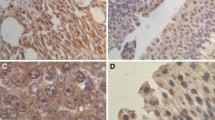
The substantial difference between normal cells and cancer cells in terms of their energy metabolism in mitochondria provides an interesting basis for the development of novel therapeutic agents targeting energy machinery of tumour cells. TOMM34 is one of the Tom (translocase of the outer membrane of mitochondria) family that was found to be overexpressed in colorectal, hepatocellular, lung and early invasive breast carcinomas. The expression profile of mitochondrial translocases in bladder cancer compared to normal urinary bladder tissues has not been investigated yet. Therefore, the aim of the current study is to investigate the expression pattern of TOMM34 in bladder cancer tissues and explore its correlation with the clinico-pathological parameters of those cases. Sixty patients who underwent either transurethral resection or radical cystectomy for bladder cancer were included in this study with revision of all their clinicopathological data and tumor slides. Ten histologically normal urothelial biopsies were also included. Immunohistochemical staining for TOMM34 was done and semi-quantitatively scored using the modified H-score. All relations were analysed using established statistical methodologies. TOMM34 overexpression was significantly associated with high tumour stage, muscle invasion and high grade. Significant positive association was observed between TOMM34 expression and poor outcome in terms of shorter disease-specific survival. This study suggests TOMM34 as a biomarker of progression and poor prognosis in urothelial cell carcinoma patients. Furthermore, we suggest a role played by mitochondrial machinery in urothelial cell carcinoma progression, which is a potential target for the newly-discovered vaccine therapy for urothelial cell carcinoma.


Similar content being viewed by others
References
Badar F, Sattar A, Meerza F et al (2009) Carcinoma of the urinary bladder in a tertiary care setting in a developing country. Asian Pac J Cancer Prev 10(3):449–452
Rogers CG, Palapattu GS, Shariat SF et al (2006) Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol 175(6):2048–2053 discussion 2053
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30
Kirkali Z, Chan T, Manoharan M et al (2005) Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66(6 Suppl 1):4–34
Stenzl A, Cowan NC, De Santis M et al (2011) Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 59(6):1009–1018
Neupert W (1997) Protein import into mitochondria. Annu Rev Biochem 66:863–917
Hoogenraad NJ, Ward LA, Ryan MT (2002) Import and assembly of proteins into mitochondria of mammalian cells. Biochim Biophys Acta 1592(1):97–105
Trachootham D, Zhang H, Zhang W et al (2008) Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood 112(5):1912–1922
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591
Lu W, Ogasawara MA, Huang P (2007) Models of reactive oxygen species in cancer. Drug Discov Today Dis Model 4(2):67–73
Chewawiwat N, Yano M, Terada K et al (1999) Characterization of the novel mitochondrial protein import component, Tom34, in mammalian cells. J Biochem 125(4):721–727
Mukhopadhyay A, Avramova LV, Weiner H (2002) Tom34 unlike Tom20 does not interact with the leader sequences of mitochondrial precursor proteins. Arch Biochem Biophys 400(1):97–104
Shimokawa T, Matsushima S, Tsunoda T et al (2006) Identification of TOMM34, which shows elevated expression in the majority of human colon cancers, as a novel drug target. Int J Oncol 29(2):381–386
Aleskandarany MA, Negm OH, Rakha EA et al (2012) TOMM34 expression in early invasive breast cancer: a biomarker associated with poor outcome. Breast Cancer Res Treat 136(2):419–427
Matsushita N, Yamamoto S, Inoue Y et al (2017) RT-qPCR analysis of the tumor antigens TOMM34 and RNF43 in samples extracted from paraffin-embedded specimens of colorectal cancer. Oncol Lett 14(2):2281–2287
McCarty KS Jr, Miller LS, Cox EB et al (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109(8):716–721
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259
de Moura MB, dos Santos LS, Van Houten B (2010) Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen 51(5):391–405
Kroemer G (2006) Mitochondria in cancer. Oncogene 25(34):4630–4632
Zhao Z, Han F, He Y et al (2014) Stromal-epithelial metabolic coupling in gastric cancer: stromal MCT4 and mitochondrial TOMM20 as poor prognostic factors. Eur J Surg Oncol 40:1361–1368
Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE et al (2012) Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle 11(7):1445–1454
Johnson JM, Lai SY, Cotzia P et al (2015) Mitochondrial metabolism as a treatment target in anaplastic thyroid cancer. Semin Oncol 42(6):915–922
Mikkilineni L, Whitaker-Menezes D, Domingo-Vidal M et al (2017) Hodgkin lymphoma: a complex metabolic ecosystem with glycolytic reprogramming of the tumormicroenvironment. Semin Oncol 44(3):218–225
Gooptu M, Whitaker-Menezes D, Sprandio J et al (2017) Mitochondrial and glycolytic metabolic compartmentalization in diffuse large B-cell lymphoma. Semin Oncol 44(3):204–217
Asmarinah A, Paradowska-Dogan A, Kodariah R et al (2014) Expression of the Bcl-2 family genes and complexes involved in the mitochondrial transport in prostate cancer cells. Int J Oncol 45(4):1489–1496
Liu Y, Lan L, Huang K et al (2014) Inhibition of Lon blocks cell proliferation, enhances chemosensitivity by promoting apoptosis and decreases cellular bioenergetics of bladder cancer potential roles of Lon as a prognostic marker and therapeutic target in bladder cancer. Oncotarget 5:11209–11224
Mo M, Peng F, Wang L et al (2013) Roles of mitochondrial transcription factor A and microRNA-590-3p in the development of bladder cancer. Oncol Lett 6:617–623
Nie X, Li M, Lu B et al (2013) Down-regulating overexpressed human Lon in cervical cancer suppresses cell proliferation and bioenergetics. PLoS One 8(11):e81084
Gibellini L, Pinti M, Boraldi F et al (2014) Silencing of mitochondrial Lon protease deeply impairs mitochondrial proteome and function in colon cancer cells. FASEB J 28(12):5122–5135
Gibellini L, Pinti M, Bartolomeo R et al (2015) Inhibition of Lon protease by triterpenoids alters mitochondria and is associated to cell death in human cancer cells. Oncotarget 6:25466–25483
Jin B, Fu G, Pan H et al (2011) Anti-tumour efficacy of mitofusin-2 in urinary bladder carcinoma. Med Oncol 28 Suppl 1:S373–S380
Oyama T, Ishikawa Y, Hayashi M et al (2007) The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer 14(2):182–188
Espina V, Wulfkuhle J, Calvert VS et al (2008) Reverse phase protein microarrays for theranostics and patient-tailored therapy. Methods Mol Biol 441:113–128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All authors have contributed significantly and are in agreement with the content of the manuscript.
Rights and permissions
About this article
Cite this article
Ahmed, M.A.H., Ali, M.H., Abbas, H.H. et al. Expression of TOMM34 and Its Clinicopathological Correlations in Urothelial Carcinoma of the Bladder. Pathol. Oncol. Res. 26, 411–418 (2020). https://doi.org/10.1007/s12253-018-0524-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0524-3