Abstract
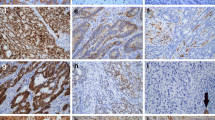
Hereditary Non-Polyposis Colorectal Cancer (HNPCC) is caused by germline mutations of mismatch-repair (MMR) genes MLH1, MSH2, MSH6 and PMS2. MLH1-/PMS2-deficient colorectal carcinomas might be HNPCC-associated but also caused by MLH1-promoter methylation in sporadic colon carcinoma. This study analyzed semiquantitatively whether the MLH1 staining pattern might be indicative of sporadic or HNPCC-associated colorectal cancer. Using a semiquantitative score ranging from 0 (negative) to 12 (maximum immunopositivity) we analyzed MLH1 expression patterns in 130 MLH1-/PMS2-deficient colorectal cancers. The collective consisted of 70 HNPCC-associated colorectal cancers and 60 sporadic colon cancers. In tumor cells of 70 HNPCC-associated colorectal cancers, 64 cases (91.43%) showed no MLH1 staining, 5 cases weak (7.14%) and 1 case (1.43%) stronger staining intensity. In contrast, in tumor cells of 60 sporadic colorectal cancers 45 cases (75.0%) showed no MLH1 staining, 10 cases weak (16.67%) and 5 cases (8.33%) stronger staining intensity to a varying extent. In immunopositive cases, MLH1 showed a characteristic dot-like nuclear staining pattern in the tumor cells. We compared cases with absent to weak MLH1-staining (immunoscores 0 to 2) to cases with elevated immunoscores (3 to 12) detecting a statistically significant difference between HNPCC-associated and sporadic colon cancers (p value = 0.0031, Fisher’s exact test). Taken together, enhanced tumoral MLH1 expression in MLH1-/PMS2-deficient colorectal carcinomas seems to be indicative of sporadic origin. In contrast, HNPCC-associated colorectal cancer showed absent or very weak MLH1 immunopositivity. Therefore, this semiquantitative and easy to exert MLH1 immunoscore might help to identify sporadic MLH1-/PMS2-deficient colorectal cancer cases prior to time-consuming methylation analysis.

Similar content being viewed by others
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691
Haggar FA, Boushey RP (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22(4):191–197
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Aran V, Victorino AP, Thuler LC, Ferreira CG (2016) Colorectal Cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer 15(3):195–203
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348(10):919–932
Peltomaki P (2003) Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 21(6):1174–1179
Cohen R, Buhard O, Cervera P, Hain E, Dumont S, Bardier A, Bachet JB, Gornet JM, Lopez-Trabada D, Dumont S, Kaci R, Bertheau P, Renaud F, Bibeau F, Parc Y, Vernerey D, Duval A, Svrcek M, André T (2017) Clinical and molecular characterisation of hereditary and sporadic metastatic colorectal cancers harbouring microsatellite instability/DNA mismatch repair deficiency. Eur J Cancer 86:266–274
Poulogiannis G, Frayling IM, Arends MJ (2010) DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 56(2):167–179
Lynch HT, Lanspa S, Shaw T, Casey MJ, Rendell M, Stacey M, Townley T, Snyder C, Hitchins M, Bailey-Wilson J (2018) Phenotypic and genotypic heterogeneity of Lynch syndrome: a complex diagnostic challenge. Familial Cancer 17(3):403–414
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I The utility of immunohistochemistry J Mol Diagn 10(4):293–300
Giuffre G et al (2005) Microsatellite analysis of hereditary nonpolyposis colorectal cancer-associated colorectal adenomas by laser-assisted microdissection: correlation with mismatch repair protein expression provides new insights in early steps of tumorigenesis. J Mol Diagn 7(2):160–170
Steinke V, Holzapfel S, Loeffler M, Holinski-Feder E, Morak M, Schackert HK, Görgens H, Pox C, Royer-Pokora B, von Knebel-Doeberitz M, Büttner R, Propping P, Engel C, on behalf of the German HNPCC Consortium (2014) Evaluating the performance of clinical criteria for predicting mismatch repair gene mutations in Lynch syndrome: a comprehensive analysis of 3,671 families. Int J Cancer 135(1):69–77
Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, Giordano F, Johnson RS, Rockwell S, Glazer PM (2003) Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol 23(9):3265–3273
Kansikas M, Kasela M, Kantelinen J, Nyström M (2014) Assessing how reduced expression levels of the mismatch repair genes MLH1, MSH2, and MSH6 affect repair efficiency. Hum Mutat 35(9):1123–1127
Kishi K, Doki Y, Yano M, Yasuda T, Fujiwara Y, Takiguchi S, Kim S, Higuchi I, Monden M (2003) Reduced MLH1 expression after chemotherapy is an indicator for poor prognosis in esophageal cancers. Clin Cancer Res 9(12):4368–4375
Mangold E, Pagenstecher C, Friedl W, Mathiak M, Buettner R, Engel C, Loeffler M, Holinski-Feder E, Müller-Koch Y, Keller G, Schackert HK, Krüger S, Goecke T, Moeslein G, Kloor M, Gebert J, Kunstmann E, Schulmann K, Rüschoff J, Propping P, the German HNPCC Consortium (2005) Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1,721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer 116(5):692–702
Gullotti L, Czerwitzki J, Kirfel J, Propping P, Rahner N, Steinke V, Kahl P, Engel C, Schüle R, Buettner R, Friedrichs N (2011) FHL2 expression in peritumoural fibroblasts correlates with lymphatic metastasis in sporadic but not in HNPCC-associated colon cancer. Lab Investig 91(12):1695–1705
Al-Nomani L et al (2015) Tumoral expression of nuclear cofactor FHL2 is associated with lymphatic metastasis in sporadic but not in HNPCC-associated colorectal cancer. Pathol Res Pract 211(2):171–174
Farchoukh L, Kuan SF, Dudley B, Brand R, Nikiforova M, Pai RK (2016) MLH1-deficient colorectal carcinoma with wild-type BRAF and MLH1 promoter Hypermethylation Harbor KRAS mutations and Arise from conventional adenomas. Am J Surg Pathol 40(10):1390–1399
Nakamura H, Tanimoto K, Hiyama K, Yunokawa M, Kawamoto T, Kato Y, Yoshiga K, Poellinger L, Hiyama E, Nishiyama M (2008) Human mismatch repair gene, MLH1, is transcriptionally repressed by the hypoxia-inducible transcription factors, DEC1 and DEC2. Oncogene 27(30):4200–4209
Sameer AS, Nissar S, Fatima K (2014) Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis. Eur J Cancer Prev 23(4):246–257
Watson P, Vasen HFA, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT, Lynch HT (2008) The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 123(2):444–449
Park JG, Vasen HF, Park Y, Park K, Peltomaki P, Ponzo de Leon M, Rodriguez-Bigas MA, Lubinski J, Beck NE, Bisgaard ML, Miyaki M, Wijnen JT, Baba S, Lindblom A, Madlensky L, Lynch HT (2002) Suspected HNPCC and Amsterdam criteria II: evaluation of mutation detection rate, an international collaborative study. Int J Color Dis 17(2):109–114
Newton K, Jorgensen NM, Wallace AJ, Buchanan DD, Lalloo F, McMahon RFT, Hill J, Evans DG (2014) Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch syndrome (HNPCC). J Med Genet 51(12):789–796
Bettstetter M, Dechant S, Ruemmele P, Vogel C, Kurz K, Morak M, Keller G, Holinski-Feder E, Hofstaedter F, Dietmaier W (2008) MethyQESD, a robust and fast method for quantitative methylation analyses in HNPCC diagnostics using formalin-fixed and paraffin-embedded tissue samples. Lab Investig 88(12):1367–1375
Yang HM, Mitchell JM, Sepulveda JL, Sepulveda AR (2015) Molecular and histologic considerations in the assessment of serrated polyps. Arch Pathol Lab Med 139(6):730–741
Mangold E, Pagenstecher C, Friedl W, Fischer HP, Merkelbach-Bruse S, Ohlendorf M, Friedrichs N, Aretz S, Buettner R, Propping P, Mathiak M (2005) Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining. J Pathol 207(4):385–395
Santos JC, Bastos AU, Cerutti JM, Ribeiro ML (2013) Correlation of MLH1 and MGMT expression and promoter methylation with genomic instability in patients with thyroid carcinoma. BMC Cancer 13:79
Springuel L, Losdyck E, Saussoy P, Turcq B, Mahon FX, Knoops L, Renauld JC (2016) Loss of mutL homolog-1 (MLH1) expression promotes acquisition of oncogenic and inhibitor-resistant point mutations in tyrosine kinases. Cell Mol Life Sci 73(24):4739–4748
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 17 kb)
Rights and permissions
About this article
Cite this article
Tarancón-Diez, M., Büttner, R. & Friedrichs, N. Enhanced Tumoral MLH1-Expression in MLH1-/PMS2-Deficient Colon Cancer Is Indicative of Sporadic Colon Cancer and Not HNPCC. Pathol. Oncol. Res. 26, 1435–1439 (2020). https://doi.org/10.1007/s12253-018-00571-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-00571-3