Abstract
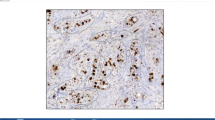
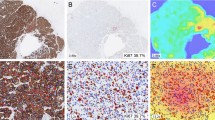
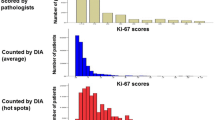
In this study, the reproducibility of Ki-67 proliferation index (KIPI) was investigated by comparing the semi-quantitative (SQ) results of three assessors with those of digital image-analysis (DIA) methods. The prognostic significance of the two approaches was also correlated with clinical outcome. Tissue microarrays of duplicate 2 mm cores were constructed from representative areas of formalin-fixed and paraffin-embedded tumor blocks of 347 breast cancer patients. SQ evaluation of Ki-67 (MIB1 clone) immunostained slides was performed independently by three pathologists. DIA was completed using a fully automated histological pattern and cell recognition module for KIPI detection (DIA-1) and an adjustable module (DIA-2) with the possibility of manual corrections. To compare SQ and DIA evaluations intra-class correlation (ICC) and concordance correlation coefficients (CCC) were determined. The three SQ evaluations demonstrated a remarkable ICC (0.853). Significant difference and poor concordance occurred between SQ-1 and SQ-2 as well as between SQ-1 and SQ-3 (p ≤ 0.001, CCC ≤ 0.827 for both comparisons). Thus, the reference KIPI value (SQ-RV) was generated from the mean values of SQ-2 and SQ-3. SQ-RV and DIA-2 results showed substantial concordance (CCC = 0.963, at p = 0.754), while SQ-RV and DIA-1 values differed (p ≤ 0.001) at only moderate concordance (CCC = 0.906). In multivariate analysis, lymph node status and SQ-2 assessment were significantly associated with clinical outcome (p ≤ 0.012 for both comparisons). Our results confirm that KIPI is a significant prognostic marker in breast cancer, which can be can be reliably reproduced by using an adjustable DIA-2 image analysis module.





Similar content being viewed by others
References
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F (2013) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi7–v23. doi:10.1093/annonc/mdt284
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31(1):13–20
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ (2015) Tailoring therapies-improving the management of early breast cancer: St Gallen international expert Consensus on the Primary therapy of early breast cancer 2015. Ann Oncol 26(8):1533–1546. doi:10.1093/annonc/mdv221
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert Consensus on the Primary therapy of early breast cancer 2013. Ann Oncol 24(9):2206–2223. doi:10.1093/annonc/mdt303
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr, American Society of Clinical Oncology (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. doi:10.1200/JCO.2007.14.2364
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF (2011) Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst 103(22):1656–1664. doi:10.1093/jnci/djr393
Polley MY, Leung SC, Gao D, Mastropasqua MG, Zabaglo LA, Bartlett JM, McShane LM, Enos RA, Badve SS, Bane AL, Borgquist S, Fineberg S, Lin MG, Gown AM, Grabau D, Gutierrez C, Hugh JC, Moriya T, Ohi Y, Osborne CK, Penault-Llorca FM, Piper T, Porter PL, Sakatani T, Salgado R, Starczynski J, Laenkholm AV, Viale G, Dowsett M, Hayes DF, Nielsen TO (2015) An international study to increase concordance in Ki67 scoring. Mod Pathol 28(6):778–786. doi:10.1038/modpathol.2015.38
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, Gown AM, Symmans WF, Piper T, Mehl E, Enos RA, Hayes DF, Dowsett M, Nielsen TO (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906. doi:10.1093/jnci/djt306
Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132(3):895–915. doi:10.1007/s10549-011-1837-z
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11(2):174–183. doi:10.1016/s1470-2045(09)70262-1
Soenksen D (2009) Digital pathology at the crossroads of major health care trends: corporate innovation as an engine for change. Arch Pathol Lab Med 133(4):555–559. doi:10.1043/1543-2165-133.4.555
Kayser K, Borkenfeld S, Kayser G (2012) How to introduce virtual microscopy (VM) in routine diagnostic pathology: constraints, ideas, and solutions. Anal Cell Pathol (Amst) 35(1):3–10. doi:10.3233/acp-2011-0044
Kayser K, Gortler J, Borkenfeld S, Kayser G (2011) How to measure diagnosis-associated information in virtual slides. Diagn Pathol 6(Suppl 1):S9. doi:10.1186/1746-1596-6-s1-s9
Laurinavicius A, Plancoulaine B, Laurinaviciene A, Herlin P, Meskauskas R, Baltrusaityte I, Besusparis J, Dasevicius D, Elie N, Iqbal Y, Bor C (2014) A methodology to ensure and improve accuracy of Ki67 labelling index estimation by automated digital image analysis in breast cancer tissue. Breast Cancer Res 16(2):R35. doi:10.1186/bcr3639
Riber-Hansen R, Vainer B, Steiniche T (2012) Digital image analysis: a review of reproducibility, stability and basic requirements for optimal results. APMIS 120(4):276–289. doi:10.1111/j.1600-0463.2011.02854.x
Ruifrok AC, Johnston DA (2001) Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23(4):291–299
Altman DG (1990) Practical statistics for medical research. Chapman and Hall, London
McBride GB (2005) A proposal for strength-of-agreement criteria for Lin’s Concordance Correlation Coefficient. NIWA Client Report: HAM2005–062
Kontzoglou K, Palla V, Karaolanis G, Karaiskos I, Alexiou I, Pateras I, Konstantoudakis K, Stamatakos M (2013) Correlation between Ki67 and breast cancer prognosis. Oncology 84(4):219–225. doi:10.1159/000346475
Mikami Y, Ueno T, Yoshimura K, Tsuda H, Kurosumi M, Masuda S, Horii R, Toi M, Sasano H (2013) Interobserver concordance of Ki67 labeling index in breast cancer: Japan breast cancer research group Ki67 ring study. Cancer Sci 104(11):1539–1543. doi:10.1111/cas.12245
Varga Z, Cassoly E, Li Q, Oehlschlegel C, Tapia C, Lehr HA, Klingbiel D, Thurlimann B, Ruhstaller T (2015) Standardization for Ki-67 assessment in moderately differentiated breast cancer. A retrospective analysis of the SAKK 28/12 study. PLoS One 10(4):e0123435. doi:10.1371/journal.pone.0123435
Cserni G, Voros A, Liepniece-Karele I, Bianchi S, Vezzosi V, Grabau D, Sapino A, Castellano I, Regitnig P, Foschini MP, Zolota V, Varga Z, Figueiredo P, Decker T, Focke C, Kulka J, Kaya H, Reiner-Concin A, Amendoeira I, Callagy G, Caffrey E, Wesseling J, Wells C (2014) Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast 23(3):259–263. doi:10.1016/j.breast.2014.02.003
Voros A, Csorgo E, Kovari B, Lazar P, Kelemen G, Rusz O, Nyari T, Cserni G (2015) Different methods of pretreatment Ki-67 labeling index evaluation in core biopsies of breast cancer patients treated with neoadjuvant chemotherapy and their relation to response to therapy. Pathol Oncol Res 21(1):147–155. doi:10.1007/s12253-014-9800-z
Maeda I, Abe K, Koizumi H, Nakajima C, Tajima S, Aoki H, Tsuchiya J, Tsuchiya S, Tsuchiya K, Shimo A, Tsugawa K, Ueno T, Tatsunami S, Takagi M (2015) Comparison between Ki67 labeling index determined using image analysis software with virtual slide system and that determined visually in breast cancer. Breast Cancer. doi:10.1007/s12282-015-0634-7
Klauschen F, Wienert S, Schmitt WD, Loibl S, Gerber B, Blohmer JU, Huober J, Rudiger T, Erbstosser E, Mehta K, Lederer B, Dietel M, Denkert C, von Minckwitz G (2015) Standardized Ki67 diagnostics using automated scoring-clinical validation in the GeparTrio breast cancer study. Clin Cancer Res 21(16):3651–3657. doi:10.1158/1078-0432.ccr-14-1283
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F (2015) Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. doi:10.1016/j.breast.2015.07.017
Joshi S, Watkins J, Gazinska P, Brown JP, Gillett CE, Grigoriadis A, Pinder SE (2015) Digital imaging in the immunohistochemical evaluation of the proliferation markers Ki67, MCM2 and Geminin, in early breast cancer, and their putative prognostic value. BMC Cancer 15:546. doi:10.1186/s12885-015-1531-3
Acknowledgements
Balazs Acs was supported by the Hungarian Talent Program, National Young Talent Award 2016 (NTP-NFTÖ-16-0496). Anna-Maria Tokes was supported by the Hungarian Society of Medical Oncology (MKOT) 2014-2016, and A. Marcell Szasz was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
We thank Gabor Kiszler Ph.D. for his innovative technical and IT support and Zsuzsanna Bodor M.D. for her excellent remarks on this study. We acknowledge Professor Zoltán Prohászka for his constructive comments on statistical analyses. We also appreciate Rita Keszthelyi’s efforts made on implementation of immunohistochemical reactions.
Author information
Authors and Affiliations
Contributions
BÁ carried out the conception and design of the study, performed digital-image analysis and statistical analyses, wrote the manuscript. LM evaluated Ki-67 IHC and revised the manuscript. KAK participated in the evaluation of Ki-67 IHC and drafted the manuscript. TM participated in digital-image analysis and helped to revise the manuscript. AMT reviewed follow-up data and drafted the manuscript. BG helped to interpret data and wrote the manuscript. JK designed and coordinated the study and revised the manuscript. AMSZ conceived of the study, and participated in its design and coordination, also evaluated Ki-67 IHC reactions, drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Institutional Review Board of Semmelweis University (IKEB, #7–1/2008). This article does not contain any studies with animals performed by any of the authors.
Informed Consent
The Institutional Review Board of Semmelweis University was consulted for patient consent requirement, written approval was unnecessary due to the nature of the study, namely routine material was utilized for the evaluation of Ki-67 without any identification of the patients and their personal data. The Helsinki Declaration of 1964 was followed in any such survey.
Rights and permissions
About this article
Cite this article
Ács, B., Madaras, L., Kovács, K.A. et al. Reproducibility and Prognostic Potential of Ki-67 Proliferation Index when Comparing Digital-Image Analysis with Standard Semi-Quantitative Evaluation in Breast Cancer. Pathol. Oncol. Res. 24, 115–127 (2018). https://doi.org/10.1007/s12253-017-0220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0220-8