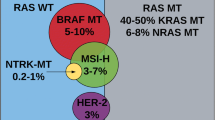
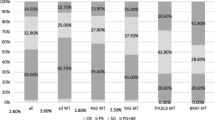
Abstract
Genetic variability in KRAS and EGFR predicts response to cetuximab in irinotecan refractory colorectal cancer. Whether these markers or others remain predictive in combination biologic therapies including bevacizumab is unknown. We identified predictive biomarkers from patients with irinotecan refractory metastatic colorectal cancer treated with cetuximab plus bevacizumab. Patients who received cetuximab plus bevacizumab for irinotecan refractory colorectal cancer in either of two Phase II trials conducted were identified. Tumor tissue was available for 33 patients. Genomic DNA was extracted and used for mutational analysis of KRAS, BRAF, and p53 genes. Fluorescence in situ hybridization was performed to assess EGFR copy number. The status of single genes and various combinations were tested for association with response. Seven of 33 patients responded to treatment. KRAS mutations were found in 14/33 cases, and 0 responded to treatment (p = 0.01). EGFR gene amplification was seen in 3/33 of tumors and in every case was associated with response to treatment (p < 0.001). TP53 and BRAF mutations were found in 18/33 and 0/33 tumors, respectively, and there were no associations with response to either gene. EGFR gene amplification and KRAS mutations are predictive markers for patients receiving combination biologic therapy of cetuximab plus bevacizumab for metastatic colorectal cancer. One marker or the other is present in the tumor of half of all patients allowing treatment response to be predicted with a high degree of certainty. The role for molecular markers in combination biologic therapy seems promising.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Macdonald JS (1999) Adjuvant therapy of colon cancer. CA Cancer J Clin 49:202–219
Meyerhardt JA, Mayer RJ (2005) Systemic therapy for colorectal cancer. N Engl J Med 352:476–487
Ruzzo A, Graziano F, Canestrari E, Magnani E (2010) Molecular predictors of efficacy to anti-EGFR agents in colorectal cancer patients. Curr Ca Drug Targets 10:68–79
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab Monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Saltz LB, Lenz HJ, Kindler HL et al (2007) Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 25(29):4557–4561
Jonker DJ, O'Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Tabernero J, Van Cutsem E, Diaz-Rubio E et al (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and Oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 25:5225–5232
Moroni M, Veronese S, Benvenuti S et al (2005) Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. The Lancet Oncology 6:279–286
Lievre A, Bachet J-B, Le Corre D et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995
Di Fiore F, Blanchard F, Charbonnier F et al (2007) Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer 96:1166–1169
Khambata-Ford S, Garrett CR, Meropol NJ et al (2007) Expression of Epiregulin and Amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237
Sartore-Bianchi A, Moroni M, Veronese S et al (2007) Epidermal growth factor receptor Gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 25(22):3238–3245
Chung KY, Shia J, Kemeny NE et al (2005) Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23:1803–1810
Shia J, Klimstra DS, Li AR et al (2005) Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Mod Pathol 18:1350–1356
Cerea G, Ricotta R, Schiavetto I et al (2006) Cetuximab for treatment of metastatic colorectal cancer. Ann Oncol 17:vii66–vii67
Khanna M, Park P, Zirvi M et al (1999) Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene 18:27–38
Sorlie T, Johnsen H, Vu P, Lind GE, Lothe R, Borresen-Dale AL (2005) Mutation screening of the TP53 gene by temporal temperature gradient gel electrophoresis. Methods Mol Biol 291:207–216
Guldberg P, Nedergaard T, Nielsen HJ, Olsen AC, Ahrenkiel V, Zeuthen J (1997) Single-step DGGE-based mutation scanning of the p53 gene: application to genetic diagnosis of colorectal cancer. Hum Mutat 9:348–355
Khan SA, Idrees K, Forslund A et al (2008) Genetic variants in germline TP53 and MDM2 SNP309 are not associated with early onset colorectal cancer. J Surg Oncol 97(7):621–625
Spindler KL, Lindebjerg J, Nielsen JN et al (2006) Epidermal growth factor receptor analyses in colorectal cancer: a comparison of methods. Int J Oncol 29(5):1159–1165
Nash GM, Gimbel M, Shia J et al (2010) KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol 17(2):572–578
Sinicrope FA, Mahoney MR, Yoon HH et al (2015) Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147 (alliance). Clin Cancer Res 21(23):5294–5304
Kato J, Futamura M, Kanematsu M et al (2016) Combination therapy with zoledronic acid and cetuximab effectively suppresses growth of colorectal cancer cells regardless or KRAS status. Int J Cancer 138(6):1516–1527
Dienstmann R, Salazer R, Tabernero J (2015) Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol 33(16):1787–1796
Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumoral heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892
Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648
Siena S, Sartre-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. (2009) Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst 101(19):1308--24
Pao W, Wang TY, Riely GJ et al (2005) KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2:e17
Lenz H-J, Van Cutsem E, Khambata-Ford S et al (2006) Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, Oxaliplatin, and Fluoropyrimidines. J Clin Oncol 24:4914–4921
Chapman SJ, McKavanagh D, Burge ME et al (2016) Effectiveness of bevacizumab and cetuximab in metastatic colorectal cancer across selected public hospitals in Queensland. Asia Pac J Clin Oncol. doi:10.1111/ajco.12518
Larsen FO, Pfeiffer P, Nielsen D et al (2011) Bevacizumab in combination with cetuximab and irinotecan after failure of cetuximab and irinotecan in patients with metastatic colorectal cancer. Acta Oncol 50(4):574–577
Acknowledgement
We thank Dr. Margaret Leversha (Cytogenetic Core Facility Lab, Memorial Sloan-Kettering Cancer Center) for valuable technical assistance for FISH analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Program Project Grant PO1-CA65930 and T32 surgical oncology training grant CA 09501 of the National Cancer Institute.
Conflict of Interest
Sajid Khan, Zhaoshi Zeng, Jinru Shia, and Philip Paty declare no conflicts of interest.
Ethical Approval
This retrospective study does not contain any studies with human participants by any of the authors. Tissue was obtained from patients enrolled in clinical trials. These trials were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Tumor block accrual were approved by the institutional review board.
Electronic supplementary material
ESM 1
(DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Khan, S.A., Zeng, Z., Shia, J. et al. EGFR Gene Amplification and KRAS Mutation Predict Response to Combination Targeted Therapy in Metastatic Colorectal Cancer. Pathol. Oncol. Res. 23, 673–677 (2017). https://doi.org/10.1007/s12253-016-0166-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0166-2