Abstract
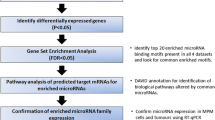
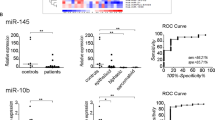
Malignant pleural mesothelioma (MPM) is the most common primary tumor of the pleura. Its incidence is still increasing in Europe and the prognosis remains poor. We investigated the oncogenic function of signal transducer and activator of transcription 1 (STAT1) in MPM in more detail. A miRNA profiling was performed on 52 MPM tissue samples. Upregulated miRNAs (targeting SOCS1/3) were knocked-down using miRNA inhibitors. mRNA expression levels of STAT1/3, SOCS1/3 were detected in MPM cell lines. STAT1 has been knocked-down using siRNA and qPCR was used to detect mRNA expression levels of all JAK/STAT family members and genes that regulate them. An immunohistochemical staining was performed to detect the expression of caspases. STAT1 was upregulated and STAT3 was downregulated, SOCS1/3 protein was not detected but it was possible to detect SOCS1/3 mRNA in MPM cell lines. The upregulated miRNAs were successfully knocked-down, however the expected effect on SOCS1 expression was not detected. STAT1 knock-down had different effects on STAT3/5 expression. Caspase 3a and 8 expression was found to be increased after STAT1 knock-down. The physiologic regulation of STAT1 via SOCS1 is completely lost in MPM and it does not seem that the miRNAs identified by now, do inhibit the expression of SOCS1. MPM cell lines compensate STAT1 knock-down by increasing the expression of STAT3 or STAT5a, two genes which are generally considered to be oncogenes. And much more important, STAT1 knock-down induces apoptosis in MPM cell lines and STAT1 might therefore be a target for therapeutic intervention.








Similar content being viewed by others
References
Vogelzang NJ, Porta C, Mutti L (2005) New agents in the management of advanced mesothelioma. Semin Oncol 32(3):336–350
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(Pt 8):1281–1283. doi:10.1242/jcs.00963
Buard A, Vivo C, Monnet I, Boutin C, Pilatte Y, Jaurand MC (1998) Human malignant mesothelioma cell growth: activation of janus kinase 2 and signal transducer and activator of transcription 1alpha for inhibition by interferon-gamma. Cancer Res 58(4):840–847
Kothmaier H, Quehenberger F, Halbwedl I, Morbini P, Demirag F, Zeren H, Comin CE, Murer B, Cagle PT, Attanoos R, Gibbs AR, Galateau-Salle F, Popper HH (2008) EGFR and PDGFR differentially promote growth in malignant epithelioid mesothelioma of short and long term survivors. Thorax 63(4):345–351. doi:10.1136/thx.2007.085241
Arzt L, Kothmaier H, Quehenberger F, Halbwedl I, Popper HH (2011) STAT signalling in malignant mesothelioma: is there a regulatory effect of microRNAs? Magazine of European. Med Oncol 4(1):38–42
Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A (2003) STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 197(2):157–168. doi:10.1002/jcp.10364
Reich NC, Liu L (2006) Tracking STAT nuclear traffic. Nat Rev Immunol 6(8):602–612. doi:10.1038/nri1885
Sadzak I, Schiff M, Gattermeier I, Glinitzer R, Sauer I, Saalmuller A, Yang E, Schaljo B, Kovarik P (2008) Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc Natl Acad Sci U S A 105(26):8944–8949. doi:10.1073/pnas.0801794105
Stephanou A, Latchman DS (2005) Opposing actions of STAT-1 and STAT-3. Growth Factors 23(3):177–182. doi:10.1080/08977190500178745
Espert L, Dusanter-Fourt I, Chelbi-Alix MK (2005) Negative regulation of the JAK/STAT: pathway implication in tumorigenesis. Bull Cancer 92(10):845–857
Wormald S, Hilton DJ (2004) Inhibitors of cytokine signal transduction. J Biol Chem 279(2):821–824. doi:10.1074/jbc.R300030200
Arzt L, Kothmaier H, Halbwedl I, Quehenberger F, Popper HH (2014) Signal transducer and activator of transcription 1 (STAT1) acts like an oncogene in malignant pleural mesothelioma. Virchows Arch 465(1):79–88. doi:10.1007/s00428-014-1584-8
Meola N, Gennarino VA, Banfi S (2009) microRNAs and genetic diseases. PathoGenetics 2(1):7. doi:10.1186/1755-8417-2-7
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR (2013) Treatment of HCV infection by targeting microRNA. N Engl J Med 368(18):1685–1694. doi:10.1056/NEJMoa1209026
Clinicaltrials.gov (2013) A Multicenter Phase I Study of MRX34. http://clinicaltrials.gov/show/NCT01829971
Ueno T, Toyooka S, Fukazawa T, Kubo T, Soh J, Asano H, Muraoka T, Tanaka N, Maki Y, Shien K, Furukawa M, Sakaguchi M, Yamamoto H, Tsukuda K, Miyoshi S (2014) Preclinical evaluation of microRNA-34b/c delivery for malignant pleural mesothelioma. Acta Med Okayama 68(1):23–26
Arzt L, Kothmaier H, Quehenberger F, Halbwedl I, Wagner K, Maierhofer T, Popper HH (2011) Evaluation of formalin-free tissue fixation for RNA and microRNA studies. Exp Mol Pathol 91(2):490–495. doi:10.1016/j.yexmp.2011.05.007
Pass HI, Stevens EJ, Oie H, Tsokos MG, Abati AD, Fetsch PA, Mew DJ, Pogrebniak HW, Matthews WJ (1995) Characteristics of nine newly derived mesothelioma cell lines. Ann Thorac Surg 59(4):835–844
Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A (1987) Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res 47(12):3199–3205
Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT (2010) Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer 9:314. doi:10.1186/1476-4598-9-314
Lesinski GB, Zimmerer JM, Kreiner M, Trefry J, Bill MA, Young GS, Becknell B, Carson WE 3rd (2010) Modulation of SOCS protein expression influences the interferon responsiveness of human melanoma cells. BMC Cancer 10:142. doi:10.1186/1471-2407-10-142
Madonna S, Scarponi C, De Pita O, Albanesi C (2008) Suppressor of cytokine signaling 1 inhibits IFN-gamma inflammatory signaling in human keratinocytes by sustaining ERK1/2 activation. FASEB J 22(9):3287–3297. doi:10.1096/fj.08-106831
Klampfer L (2006) Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets 6(2):107–121
Iwahori K, Serada S, Fujimoto M, Ripley B, Nomura S, Mizuguchi H, Shimada K, Takahashi T, Kawase I, Kishimoto T, Naka T (2013) SOCS-1 gene delivery cooperates with cisplatin plus pemetrexed to exhibit preclinical antitumor activity against malignant pleural mesothelioma. Int J Cancer 132(2):459–471. doi:10.1002/ijc.27611
Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302(1):1–12. doi:10.1016/j.ydbio.2006.08.028
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103(7):2257–2261. doi:10.1073/pnas.0510565103
Busacca S, Germano S, De Cecco L, Rinaldi M, Comoglio F, Favero F, Murer B, Mutti L, Pierotti M, Gaudino G (2010) MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol 42(3):312–319. doi:10.1165/rcmb.2009-0060OC
Reid G, Pel ME, Kirschner MB, Cheng YY, Mugridge N, Weiss J, Williams M, Wright C, Edelman JJ, Vallely MP, McCaughan BC, Klebe S, Brahmbhatt H, MacDiarmid JA, van Zandwijk N (2013) Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 24(12):3128–3135. doi:10.1093/annonc/mdt412
Truini A, Coco S, Alama A, Genova C, Sini C, Dal Bello MG, Barletta G, Rijavec E, Burrafato G, Boccardo F, Grossi F (2014) Role of microRNAs in malignant mesothelioma. Cell Mol life Sci: CMLS. doi:10.1007/s00018-014-1584-5
Qing Y, Stark GR (2004) Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem 279(40):41679–41685. doi:10.1074/jbc.M406413200
NCBI F2R coagulation factor II (thrombin) receptor http://www.ncbi.nlmnih.gov/gene/2149
NCBI JUN proto-oncogene. http://www.ncbi.nlmnih.gov/gene/3725
NCBI CDKN1A cyclin-dependent kinase inhibitor 1A (p21, Cip1). http://www.ncbi.nlmnih.gov/gene/1026
Romanov VS, Pospelov VA, Pospelova TV (2012) Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on its role in senescence and oncogenesis. Biochemistry. Biokhimiia 77(6):575–584. doi:10.1134/S000629791206003X
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75(4):817–825
Lohr K, Moritz C, Contente A, Dobbelstein M (2003) p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem 278(35):32507–32516. doi:10.1074/jbc.M212517200
Wang Y, Blandino G, Givol D (1999) Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene 18(16):2643–2649. doi:10.1038/sj.onc.1202632
Adams JM, Cory S (2002) Apoptosomes: engines for caspase activation. Curr Opin Cell Biol 14(6):715–720
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424. doi:10.1146/annurev.biochem.68.1.383
Acknowledgements
We gratefully thank Hildegard Gleixner, Virginia Brenner, Elisabeth Grygar and Mohammed Al-Effah, Institute of Pathology, Medical University of Graz, Karin Wagner and Theresa Maierhofer, Center for Medical Research, Medical University of Graz, Franz Quehenberger, Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz as well as Thomas Mauthner, Institute for Computer Graphics and Vision, Graz University of Technology for excellent technical assistance.
The study was supported by an unrestricted grant from Pfizer Austria.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 91 kb)
Rights and permissions
About this article
Cite this article
Arzt, L., Halbwedl, I., Gogg-Kamerer, M. et al. Signal Transducer and Activator of Transcription 1 (STAT1) Knock-down Induces Apoptosis in Malignant Pleural Mesothelioma. Pathol. Oncol. Res. 23, 595–605 (2017). https://doi.org/10.1007/s12253-016-0157-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0157-3