Abstract
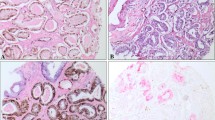
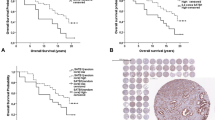
SPINK1 is proposed as potential prognostic marker in prostate cancer (PCA). However, its relation to PTEN and ERG in localized PCA remains unclear. The study population consisted of two independent cohorts of men treated by radical prostatectomy for localized PCA (discovery n = 218 and validation n = 129). Patterns of association between SPINK1 and each of ERG and PTEN were evaluated by immunohistochemistry and fluorescence in situ hybridization. Associations between SPINK1 expression and various pathologic parameters and clinical outcome were also investigated. SPINK1 was expressed in 15.3 % and 10.9 % of cases in the discovery and validation cohort, respectively. SPINK expression was observed in 5.56 % of high-grade prostatic intraepithelial neoplasia and 1.1 % of adjacent morphologically benign prostatic glands. SPINK1 and ERG expression were almost exclusive, with only 1.0 % of the cases co-expressing both in the same core sample. SPINK1 interfocal and within-core heterogeneity was noted in 29.2 % and 64.6 % of cases, respectively. SPINK1 expression was not significantly associated with PTEN deletion in the two cohorts (p = 0.871 for discovery cohort and p = 0.293 for validation cohort). While SPINK1 expression did occur with hemizygous PTEN deletion, there was a complete absence of SPINK1 expression in PCA showing homozygous PTEN deletion, which was confirmed in the validation cohort (p = 0.02). Despite SPINK1’s association with higher Gleason score (>7) (p = 0.02), it was not associated with other pathological parameters or biochemical recurrence post-radical prostatectomy. We documented absolute exclusivity between SPINK1 overexpression and homozygous PTEN deletion in localized PCA. SPINK1 and ERG expressions are exclusive events in PCA. SPINK1 is not of added prognostic value in localized PCA.




Similar content being viewed by others
Abbreviations
- BAC:
-
Bacterial artificial chromosome
- CRPC:
-
Castrate resistant prostate cancer
- DAB:
-
3,3′-Diaminobenzidine (DAB)
- ERG:
-
ETS-related gene
- FISH:
-
Fluorescence in-situ hybridization
- GS(s):
-
Gleason score(s)
- H&E:
-
Haematoxylin and eosin
- HGPIN(s):
-
High-grade prostatic intraepithelial neoplasia(s)
- IHC:
-
Immunohistochemistry
- KDal:
-
Kilodalton
- PCA(s):
-
Prostate cancer(s)
- PSA:
-
Prostate-specific antigen
- PSTI:
-
Pancreatic secretory trypsin inhibitor
- PTEN:
-
Phosphatase and tensin homolog
- SD:
-
Standard deviations
- SPINK1:
-
Serine protease inhibitor Kazal-type 1
- TATI:
-
Tumour-associated trypsin inhibitor
- TMA:
-
Tissue microarray
- TMPRSS2:
-
Transmembrane protease, serine 2
- UHN:
-
University Health Network
References
Horii A, Kobayashi T, Tomita N, Yamamoto T, Fukushige S, Murotsu T et al (1987) Primary structure of human pancreatic secretory trypsin inhibitor (PSTI) gene. Biochem Biophys Res Commun 149(2):635–641
Stenman UH (2002) Tumor-associated trypsin inhibitor. Clin Chem 48(8):1206–1209
Flavin R, Pettersson A, Hendrickson WK, Fiorentino M, Finn S, Kunz L et al (2014) SPINK1 protein expression and prostate cancer progression. Clin Cancer Res 20(18):4904–4911. doi:10.1158/1078-0432.CCR-13-1341
Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S et al (2008) The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell 13(6):519–528. doi:10.1016/j.ccr.2008.04.016
Bhalla R, Kunju LP, Tomlins SA, Christopherson K, Cortez C, Carskadon S et al (2013) Novel dual-color immunohistochemical methods for detecting ERG-PTEN and ERG-SPINK1 status in prostate carcinoma. Mod Pathol 26(6):835–848. doi:10.1038/modpathol.2012.234
Leinonen KA, Saramaki OR, Furusato B, Kimura T, Takahashi H, Egawa S et al. (2013). Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 22(12):2333–44. doi:10.1158/1055-9965.EPI-13-0333-T.
Jhavar S, Brewer D, Edwards S, Kote-Jarai Z, Attard G, Clark J et al (2009) Integration of ERG gene mapping and gene-expression profiling identifies distinct categories of human prostate cancer. BJU Int 103(9):1256–1269. doi:10.1111/j.1464-410X.2008.08200.x
Lippolis G, Edsjo A, Stenman UH, Bjartell A (2013) A high-density tissue microarray from patients with clinically localized prostate cancer reveals ERG and TATI exclusivity in tumor cells. Prostate Cancer Prostatic Dis 16(2):145–150. doi:10.1038/pcan.2013.7
Wang C, Wang L, Su B, Lu N, Song J, Yang X et al (2014) Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate 74(7):689–701. doi:10.1002/pros.22787
Leinonen KA, Tolonen TT, Bracken H, Stenman UH, Tammela TL, Saramaki OR et al (2010) Association of SPINK1 expression and TMPRSS2:ERG fusion with prognosis in endocrine-treated prostate cancer. Clin Cancer Res 16(10):2845–2851. doi:10.1158/1078-0432.CCR-09-2505
Grupp K, Diebel F, Sirma H, Simon R, Breitmeyer K, Steurer S et al (2013) SPINK1 expression is tightly linked to 6q15- and 5q21-deleted ERG-fusion negative prostate cancers but unrelated to PSA recurrence. Prostate 73(15):1690–1698. doi:10.1002/pros.22707
Terry S, Nicolaiew N, Basset V, Semprez F, Soyeux P, Maille P et al (2015) Clinical value of ERG, TFF3, and SPINK1 for molecular subtyping of prostate cancer. Cancer 121(9):1422–1430. doi:10.1002/cncr.29233
Brooks JD, Wei W, Hawley S, Auman H, Newcomb L, Boyer H et al (2015) Evaluation of ERG and SPINK1 by immunohistochemical staining and Clinicopathological outcomes in a multi-institutional radical prostatectomy cohort of 1067 patients. PLoS One 10(7):e0132343. doi:10.1371/journal.pone.0132343
Schrecengost R, Knudsen KE (2013) Molecular pathogenesis and progression of prostate cancer. Semin Oncol 40(3):244–258. doi:10.1053/j.seminoncol.2013.04.001
Bismar TA, Yoshimoto M, Duan Q, Liu S, Sircar K, Squire JA (2012) Interactions and relationships of PTEN, ERG, SPINK1 and AR in castration-resistant prostate cancer. Histopathology 60(4):645–652. doi:10.1111/j.1365-2559.2011.04116.x
Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA et al (2007) FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer 97(5):678–685
Epstein JI, Allsbrook WC, Jr., Amin MB, Egevad LL, Committee IG. (2005). The 2005 international society of urological pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol, 29(9):1228–1242.
Huang KC, Alshalalfa M, Hegazy SA, Dolph M, Donnelly B, Bismar TA (2014) The prognostic significance of combined ERG and androgen receptor expression in patients with prostate cancer managed by androgen deprivation therapy. Cancer Biol Ther 15(9):1120–1128. doi:10.4161/cbt.29689
Yoshimoto M, Ding K, Sweet JM, Ludkovski O, Trottier G, Song KS et al (2013) PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Mod Pathol: an official J US Can Acad Pathol, Inc 26(3):435–447. doi:10.1038/modpathol.2012.162
Teng LH, Wang C, Begin LR, Dolph M, Yilmaz A, Trpkov K et al (2013) ERG protein expression and gene rearrangements are present at lower rates in metastatic and locally advanced castration-resistant prostate cancer compared to localized disease. Urology 82(2):394–399. doi:10.1016/j.urology.2013.03.029
Braun M, Goltz D, Shaikhibrahim Z, Vogel W, Bohm D, Scheble V et al (2012) ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer--a comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis 15(2):165–169. doi:10.1038/pcan.2011.67
Smith SC, Tomlins SA (2014) Prostate cancer SubtyPINg biomarKers and outcome: is clarity emERGing? Clin Cancer Res 20(18):4733–4736. doi:10.1158/1078-0432.CCR-14-0818
Smith SC, Palanisamy N, Zuhlke KA, Johnson AM, Siddiqui J, Chinnaiyan AM et al (2014) HOXB13 G84E-related familial prostate cancers: a clinical, histologic, and molecular survey. Am J Surg Pathol 38(5):615–626. doi:10.1097/PAS.0000000000000090
Gumuskaya B, Gurel B, Fedor H, Tan HL, Weier CA, Hicks JL et al (2013) Assessing the order of critical alterations in prostate cancer development and progression by IHC: further evidence that PTEN loss occurs subsequent to ERG gene fusion. Prostate Cancer Prostatic Dis 16(2):209–215. doi:10.1038/pcan.2013.8
Acknowledgment
This work was supported in part by the Prostate Cancer Foundation Young Investigator Award (T.A.B). This work was also supported by Prostate cancer Canada and is proudly funded by the Movember Foundation-Grant #B2013-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
The authors have no conflict of interest to declare in this study.
Electronic supplementary material
Supplementary figure 1
PTEN genomic deletions using four-colour interphase FISH strategy. A. Wild type PTEN; B. Hemizygous deletions and C. Homozygous deletion (GIF 299 kb)
Supplementary figure 2
Morphologically benign gland showing SPINK1 expression on IHC (original magnification 200×) (GIF 670 kb)
Rights and permissions
About this article
Cite this article
Huang, KC., Evans, A., Donnelly, B. et al. SPINK1 Overexpression in Localized Prostate Cancer: a Rare Event Inversely Associated with ERG Expression and Exclusive of Homozygous PTEN Deletion. Pathol. Oncol. Res. 23, 399–407 (2017). https://doi.org/10.1007/s12253-016-0119-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0119-9