Abstract
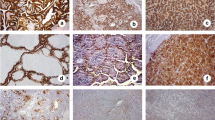
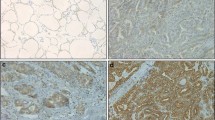
Ephrin receptors (Ephs) are frequently overexpressed in a wide variety of human malignant tumors, being associated with tumor growth, invasion, angiogenesis and metastasis. The present study aimed to evaluate the clinical significance of EphB4 and EphB6 protein expression in human malignant and benign thyroid lesions. EphB4 and EphB6 protein expression was assessed immunohistochemically on paraffin-embedded thyroid tissues obtained from 127 patients with benign (n = 71) and malignant (n = 56) thyroid lesions. Enhanced EphB4 and EphB6 expression was more frequently observed in malignant compared to benign thyroid lesions (p = 0.0508 and p = 0.0006, respectively). EphB4 and EphB6 expression also provided a distinct discrimination between papillary carcinoma and hyperplastic nodules (p = 0.0302 and p = 0.0013, respectively). In malignant thyroid lesions, enhanced EphB4 expression was significantly associated with larger tumor size (p = 0.0366). Enhanced EphB6 expression was significantly associated with larger tumor size (p = 0.0366), the presence of lymph node metastases (p = 0.0023), the presence of capsular (p = 0.0038), lymphatic (p = 0.0053) and vascular invasion (p = 0.0018) and increased risk of recurrence rate (p = 0.0038). The present study supported evidence that EphB4 and mainly EphB6 may participate in the malignant thyroid transformation, reinforcing their utility as useful biomarkers and possible therapeutic targets in this type of neoplasia.

Similar content being viewed by others
References
Zhang J, Hughes SE (2006) Role of the ephrin and Ephrin receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol 208:453–461
Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133:38–52
Nakamoto M, Bergemann AD (2002) Diverse role for the Eph family of receptor tyrosine kinases in carcinogenesis. Microscopy Res Technique 59:58–67
Surawska H, Ma PC, Salgia R (2004) The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev 15:419–433
Cheng N, Brantley DM, Chen J (2002) The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev 13:75–85
Brandley-Sieders DM, Chen J (2007) Eph receptor tyrosine kinase in angiogenesis: from development to disease. Angiogenesis 7:17–28
Castaño J, Davalos V, Schwartz S Jr, Arango D (2008) EPH receptors in cancer. Histol Histopathol 23:1011–1023
Leenhardt L, Grosclaude P, Cherie-Challine L (2004) Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14:1056–1060
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Hundahl SA, Fleming ID, Fremgen AM et al (1998) A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648
Gharib H, Papini E (2007) Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 36:707–735
Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM (2007) Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat Clin Pract Endocrinol Metab 3:260–269
Stang MT, Carty SE (2009) Recent developments in predicting thyroid malignancy. Curr Opin Oncol 21:11–17
Fischer S, Asa L (2008) Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med 132:359–372
Vriens MR, Schreinemakers JM, Suh I et al (2009) Diagnostic markers and prognostic factors in thyroid cancer. Future Oncol 5:1283–1293
Aisner DL, Sams SB (2012) The role of cytology specimens in molecular testing of solid tumors: techniques, limitations, and opportunities. Diagn Cytopathol 40:511–524
Giaginis C, Tsourouflis G, Zizi-Serbetzoglou A, Kouraklis G, Chatzopoulou E, Theocharis S (2010) Clinical significance of Ephrin (Eph)-A1, −A2, −A4, −A5 and -A7 receptors in pancreatic ductal adenocarcinoma. Pathol Oncol Res 16:267–276
Giaginis C, Tsoukalas N, Bournakis E et al (2014) Ephrin (Eph) receptor A1, A4, A5 and A7 expression in human non-small cell lung carcinoma: associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Clin Pathol 14:8
Karidis NP, Giaginis C, Tsourouflis G, Alexandrou P, Delladetsima I, Theocharis S (2011) Eph-A2 and Eph-A4 expression in human benign and malignant thyroid lesions: an immunohistochemical study. Med Sci Monit 17:BR257–BR265
Theocharis S, Klijanienko J, Giaginis C, Alexandrou P, Patsouris E, Sastre-Garau X (2013) Ephrin receptor (Eph) -A1, −A2, −A4 and -A7 expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patients survival. Pathol Oncol Res. doi:10.1007/s12253-013-9692-3
Yin H, Lu C, Tang Y, Wang H, Wang H, Wang J (2013) Enhanced expression of EphrinB1 is associated with lymph node metastasis and poor prognosis in breast cancer. Cancer Biomark 13:261–267
Ji XD, Li G, Feng YX et al (2011) EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res 71:1156–1166
Li M, Zhao ZW, Zhang Y, Xin Y (2011) Over-expression of Ephb4 is associated with carcinogenesis of gastric cancer. Dig Dis Sci 56:698–706
Lu Z, Zhang Y, Li Z et al (2012) Overexpression of the B-type Eph and ephrin genes correlates with progression and pain in human pancreatic cancer. Oncol Lett 3:1207–1212
Oshima T, Akaike M, Yoshihara K et al (2008) Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int J Oncol 33:573–577
Wang H, Wen J, Wang H et al (2013) Loss of expression of EphB1 protein in serous carcinoma of ovary associated with metastasis and poor survival. Int J Clin Exp Pathol 7:313–321
Xuqing W, Lei C, Zhengfa M et al (2012) EphB4 is overexpressed in papillary thyroid carcinoma and promotes the migration of papillary thyroid cancer cells. Tumour Biol 33:1419–1427
Tang XX, Zhao H, Robinson ME et al (2000) Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proc Natl Acad Sci U S A 97:10936–10941
Hafner C, Bataille F, Meyer S et al (2003) Loss of EphB6 expression in metastatic melanoma. Int J Oncol 23:1553–1559
Rosai J, Appendix C (2004) Staging of cancer. In: Houston M (ed) Rosai and Ackerman’s surgical pathology, 9th edn. Mosby, London, pp 2809–2810
Tuttle RM, Tala H, Shah J et al (2010) Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341
Tu Y, He S, Fu J et al (2012) Expression of EphrinB2 and EphB4 in glioma tissues correlated to the progression of glioma and the prognosis of glioblastoma patients. Clin Transl Oncol 14:214–220
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T (2009) Coexpression of EphB4 and ephrinB2 in tumor advancement of uterine cervical cancers. Gynecol Oncol 114:84–98
Zheng MF, Ji Y, Wu XB, Ye SG, Chen JY (2012) EphB4 gene polymorphism and protein expression in non-small-cell lung cancer. Mol Med Rep 6:405–408
Sinha UK, Mazhar K, Chinn SB et al (2006) The association between elevated EphB4 expression, smoking status, and advanced-stage disease in patients with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 132:1053–1059
Davalos V, Dopeso H, Castaño J et al (2006) EPHB4 and survival of colorectal cancer patients. Cancer Res 66:8943–8948
Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I (2001) Expression of receptor tyrosine kinase EphB4 and its ligand ephrin-B2 is associated with malignant potential in endometrial cancer. Oncol Rep 8:567–573
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T (2008) Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br J Cancer 98:845–851
Tang XX, Evans AE, Zhao H et al (1999) High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res 5:1491–1496
Xi HQ, Wu XS, Wei B, Chen L (2012) Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med 16:2894–2909
Ferguson BD, Liu R, Rolle CE et al (2013) The EphB4 receptor tyrosine kinase promotes lung cancer growth: a potential novel therapeutic target. PLoS One 8:e67668
Hasina R, Mollberg N, Kawada I et al (2013) Critical role for the receptor tyrosine kinase EPHB4 in esophageal cancers. Cancer Res 73:184–194
Spannuth WA, Mangala LS, Stone RL et al (2010) Converging evidence for efficacy from parallel EphB4-targeted approaches in ovarian carcinoma. Mol Cancer Ther 9:2377–2388
Conflicts of interest
All authors verify that they have not accepted any funding or support from an organization that may in any way gain or lose financially from the results of the present study. All authors verify that they have not been employed by an organization that may in any way gain or lose financially from the results of the present study. None authors have any other conflicting interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giaginis, C., Alexandrou, P., Poulaki, E. et al. Clinical Significance of EphB4 and EphB6 Expression in Human Malignant and Benign Thyroid Lesions. Pathol. Oncol. Res. 22, 269–275 (2016). https://doi.org/10.1007/s12253-014-9879-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-014-9879-2