Abstract
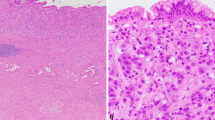
ROS produced from Oxidative stress have long been recognized to be involved in carcinogenesis. p66Shc generates H2O2 by oxidizing cytochrome c, and its expression has been reported to be elevated in several tumors. However, the expression of p66Shc in gastric cancer has not been reported, and its role in colorectal cancer has not been well elucidated. This study investigated p66Shc expression in benign, premalignant, and malignant gastric and colorectal lesions. p66Shc expression in 146 gastric tumors, 136 colorectal tumors, 45 gastric hyperplastic polyps, 33 gastric low-grade intraepithelial neoplasias, 41 gastric high-grade intraepithelial neoplasias, 42 colorectal hyperplastic polyps, 21 colorectal low-grade intraepithelial neoplasias, 38 colorectal high-grade intraepithelial neoplasias, and 30 normal gastric and colorectal tissues was measured by immunohistochemistry. Most normal gastric and colorectal tissues exhibited low or no p66Shc expression (93.4 %), while most gastric and colorectal tumors exhibited moderate to high p66Shc expression (78.1 %–80.9 %). The p66Shc expression in normal gastric and colorectal tissues were significantly lower than that in the low-grade intraepithelial neoplasias (p < 0.05), high-grade intraepithelial neoplasias (p < 0.01), and gastric adenocarcinomas (p < 0.01 or <0.001). No differences in p66Shc expression were observed in gastric and colorectal hyperplastic polyps compared to the normal tissues. No statistically significant differences in p66Shc expression were observed between patients with different disease stages, different tumor grades, and with or without lymph node metastasis in gastric and colorectal cancers. In conclusion, p66Shc may be involved in the carcinogenesis of gastric and colorectal cancers and could be a marker for the diagnosis of gastric and colorectal cancers.


Similar content being viewed by others
References
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11:777–790
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Storz P (2005) Reactive oxygen species in tumor progression. Front Biosci 10:1881–1896
Oberley LW (2005) Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed Pharmacother 59:143–148
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313:17–29
Leto TL, Geiszt M (2006) Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal 8:1549–1561
Yoshida S, Masaki T, Feng H, Yuji J, Miyauchi Y, Funaki T et al (2004) Enhanced expression of adaptor molecule p46 Shc in nuclei of hepatocellular carcinoma cells: study of LEC rats. Int J Oncol 25:1089–1096
Ravichandran KS (2001) Signaling via Shc family adapter proteins. Oncogene 20:6322–6330
Rajendran M, Thomes P, Zhang L, Veeramani S, Lin MF (2010) p66Shc–a longevity redox protein in human prostate cancer progression and metastasis: p66Shc in cancer progression and metastasis. Cancer Metastasis Rev 29:207–222
Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP et al (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313
Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C et al (2005) Electron transfer between cytochrome c and p66 Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233
Jackson JG, Yoneda T, Clark GM, Yee D (2000) Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clin Cancer Res 6:1135–1139
Abdollahi A, Gruver BN, Patriotis C, Hamilton TC (2003) Identification of epidermal growth factor-responsive genes in normal rat ovarian surface epithelial cells. Biochem Biophys Res Commun 18:188–197
Park YJ, Kim TY, Lee SH, Kim H, Kim SW, Shong M et al (2005) p66shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology 146:2473–2480
Galimov ER, Sidorenko AS, Tereshkova AV, Pletiushkina OI, Cherniak BV, Chumakov PM (2012) P66shc action on resistance of colon carcinoma RKO cells to oxidative stress. Mol Biol (Mosk) 46:139–146
Grossman SR, Lyle S, Resnick MB, Sabo E, Lis RT, Rosinha E et al (2007) p66 Shc tumor levels show a strong prognostic correlation with disease outcome in stage IIA colon cancer. Clin Cancer Res 13:5798–5804
Lee MS, Igawa T, Chen SJ, Van Bemmel D, Lin JS, Lin FF et al (2004) p66Shc protein is upregulated by steroid hormones in hormone-sensitive cancer cells and in primary prostate carcinomas. Int J Cancer 108:672–678
Hamilton SR, Aaltonen LA (2000) WHO classification of tumours: pathology and genetics of tumours of the digestive system. IARC Press, Lyon
Dango S, Sienel W, Schreiber M, Stremmel C, Kirschbaum A, Pantel K, Passlick B (2008) Elevated expression of carcino-embryonic antigen-related cell adhesionmolecule 1 (CEACAM-1) is associated with increased angiogenic potential in non-small-cell lung cancer. Lung Cancer 60:426–433
Yukimasa S, Masaki T, Yoshida S, Uchida N, Watanabe S, Usuki H, Yoshiji H, Maeta T, Ebara K, Nakatsu T, Kurokohchi K, Kuriyama S (2005) Enhanced expression of p46 Shc in the nucleus and p52 Shc in the cytoplasm of human gastric cancer. Int J Oncol 26:905–911
Ventura A, Luzi L, Pacini S, Baldari CT, Pelicci PG (2002) The p66Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. J Biol Chem 277:22370–22376
Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E et al (2002) Ap53-p66Shc signalling pathway controls intracellular redox status, levels of oxidationdamagedDNAand oxidative stress-induced apoptosis. Oncogene 21:3872–3878
Pellegrini M, Pacini S, Baldari CT (2005) p66SHC: the apoptotic side of Shc proteins. Apoptosis 10:13–18
Pani G, Koch OR, Galeotti T (2009) The p53-p66shc-Manganese Superoxide Dismutase (MnSOD) network: a mitochondrial intrigue to generate reactive oxygen species. Int J Biochem Cell Biol 41:1002–1005
Nicco C, Laurent A, Chereau C, Weill B, Batteux F (2005) Differential modulation of normal and tumor cell proliferation by reactive oxygen species. Biomed Pharmacother 59:169–174
Xiao D, Singh SV (2010) p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Res 70:3150–3158
Conflict of Interest
All authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, G., Xie, B., Gong, L. et al. The Expression of p66Shc Protein in Benign, Premalignant, and Malignant Gastrointestinal Lesions. Pathol. Oncol. Res. 20, 733–739 (2014). https://doi.org/10.1007/s12253-014-9754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-014-9754-1