Abstract
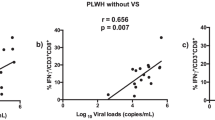
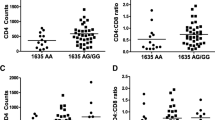
Although antiretroviral treatment lowers the burden of human immunodeficiency virus (HIV)-related disease, it does not always result in immunological recovery. This manifests as persistent chronic inflammation, immune activation or exhaustion that can promote the onset of co-morbidities. As the exact function of regulatory T (Treg) cells in HIV remains unclear, this cross-sectional study investigated three expression markers (Forkhead box protein P3 [FOXP3], glycoprotein A repetitions predominant [GARP], special AT-rich sequence binding protein 1 [SATB1]) and compared their expansion between CD4+CD25− and CD4+CD25++ T cells. Age-matched study subjects were recruited (Western Cape, South Africa) and sub-divided: HIV-negative subjects (n = 12), HIV-positive naïve treated (n = 22), HIV-positive treated based on CD4 count cells/µL (CD4 > 500 and CD4 < 500) (n = 34) and HIV-treated based on viral load (VL) copies/mL (VL < 1000 and VL > 1000) (n = 34). Markers of immune activation (CD38) and coagulation (CD142) on T cells (CD8) were assessed by flow cytometry together with FOXP3, GARP and SATB1 expression on CD4+CD25− and CD4+CD25++ T cells. Plasma levels of interleukin-10 (IL-10; anti-inflammatory marker), IL-6 (inflammatory marker) and D-dimer (coagulation marker) were assessed. This study revealed three major findings in immuno-compromised patients with virological failure (CD4 < 500; VL > 1000): (1) the expansion of the unconventional Treg cell subset (CD4+CD25−FOXP3+) is linked with disease progression markers; (2) increased GARP expression in the CD4+CD25− and CD4+CD25++ subsets; and (3) the identification of a strong link between CD4+CD25−SATB1+ cells and markers of immune activation (CD8+CD38+) and coagulation (CD8+CD142+ and D-dimer).




Similar content being viewed by others
References
Amarnath S, Dong L, Li J, Wu Y, Chen W (2007) Endogenous TGF-β activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology 4:57
Angerami MT, Suarez GV, Vecchione MB, Laufer N, Ameri D, Ben G, Perez H, Sued O, Salomón H, Quiroga MF (2017) Expansion of CD25-negative forkhead box P3-positive T cells during HIV and mycobacterium tuberculosis infection. Front Immunol 8:528
Beyer M, Thabet Y, Müller RU, Sadlon T, Classen S, Lahl K, Basu S, Zhou X, Bailey-Bucktrout SL, Krebs W et al (2011) Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol 12:898–907
Brenchley JM, Douek DC (2008) HIV infection and the gastrointestinal immune system. Mucosal Immunol 1:23–30
Catalfamo M, Le Saout C, Lane HC (2012) The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev 23:207–214
Chevalier MF, Petitjean G, Dunyach-Rémy C, Didier C, Girard PM, Manea ME, Campa P, Meyer L, Rouzioux C, Lavigne JP, Barré-Sinoussi F, Scott-Algara D, Weiss L (2013) The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog 9:e1003453
Chevalier MF, Weiss L (2013) The split personality of regulatory T cells in HIV infection. Blood 121:29–37
Dominick L, Midgley N, Swart LM, Sprake D, Deshpande G, Laher I, Joseph D, Teer E, Essop MF (2020) HIV-related cardiovascular diseases: the search for a unifying hypothesis. Am J Physiol - Hear Circ Physiol 318:H731–H746
Elkord E, Abd Al Samid M, Chaudhary B (2015) Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget 6:20026–20036
Fan MY, Low JS, Tanimine N, Finn KK, Priyadharshini B, Germana SK, Kaech SM, Turka LA (2018) Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep 25:1204–1213
Février M, Dorgham K, Rebollo A (2011) CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses 3:586–612
Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J (2007) Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 5:e38
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336
Furtado GC, De Curotto Lafaille MA, Kutchukhidze N, Lafaille JJ (2002) Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med 196:851–857
Grzanka J, Leveson-Gower D, Golab K, Wang XJ, Marek-Trzonkowska N, Krzystyniak A, Wardowska A, Mills JM, Trzonkowski P, Witkowski P (2013) FoxP3, Helios, and SATB1: roles and relationships in regulatory T cells. Int Immunopharmacol 16:343–347
Horwitz DA (2010) Identity of mysterious CD4+CD25-Foxp3+ cells in SLE. Arthritis Res Ther 12:101
Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR (2008) The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis 46:1046–1052
Kleinman AJ, Sivanandham R, Pandrea I, Chougnet CA, Apetrei C (2018) Regulatory T cells as potential targets for HIV cure research. Front Immunol 9:734
Kondo M, Tanaka Y, Kuwabara T, Naito T, Kohwi-Shigematsu T, Watanabe A (2016) SATB1 plays a critical role in establishment of immune tolerance. J Immunol 196:563–572
Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD; INSIGHT SMART Study Group (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5:e203
Metelli A, Salem M, Wallace CH, Wu BX, Li A, Li X, Li Z (2018) Immunoregulatory functions and the therapeutic implications of GARP-TGF-β in inflammation and cancer. J Hematol Oncol 11:24
Miller MM, Fogle JE, Ross P, Tompkins MB (2013) Feline glycoprotein A repetitions predominant anchors transforming growth factor beta on the surface of activated CD4(+)CD25(+) regulatory T cells and mediates AIDS lentivirus-induced T cell immunodeficiency. AIDS Res Hum Retroviruses 29:641–651
Miller MM, Petty CS, Tompkins MB, Fogle JE (2014) CD4+CD25+ T regulatory cells activated during feline immunodeficiency virus infection convert T helper cells into functional suppressors through a membrane-bound TGFβ / GARP-mediated mechanism. Virol J 11:7
Min B (2017) Heterogeneity and Stability in Foxp3+ regulatory T cells. J Interferon Cytokine Res 37:386–397
Moreno-Fernandez ME, Presicce P, Chougnet CA (2012) Homeostasis and function of regulatory T cells in HIV/SIV infection. J Virol 86:10262–10269
Naicker DD, Werner L, Kormuth E, Passmore JA, Mlisana K, Karim SA, Ndung'u T; CAPRISA Acute Infection Study Team (2009) Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J Infect Dis 200:448–452
Nou E, Lo J, Grinspoon SK (2016) Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 30:1495–1509
Paiardini M, Müller-Trutwin M (2013) HIV-associated chronic immune activation. Immunol Rev 254:78–101
Prabhakar B, Banu A, Pavithra HB, Chandrashekhara P, Sasthri S (2011) Immunological failure despite virological suppression in HIV seropositive individuals on antiretroviral therapy. Indian J Sex Transm Dis AIDS 32:94–98
Probst-Kepper M, Buer J (2010) FOXP3 and GARP (LRRC32): the master and its minion. Biol Direct 5:8
Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR (2013) Persistent immune activation in chronic HIV infection: do any interventions work? AIDS 27:1199–1208
Rojas JM, Avia M, Martín V, Sevilla N (2017) IL-10: a multifunctional cytokine in viral infections. J Immunol Res 2017:1–14
Stockis J, Colau D, Coulie PG, Lucas S (2009) Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol 39:3315–3322
Stylianou E, Aukrust P, Kvale D, Müller F, Frøland SS (1999) IL-10 in HIV infection: increasing serum IL-10 levels with disease progression-down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol 116:115–120
Suchard MS, Mayne E, Green VA, Shalekoff S, Donninger SL, Stevens WS, Gray CM, Tiemessen CT (2010) FOXP3 expression is upregulated in CD4+T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS ONE 5:e11762
Teer E, Joseph DE, Driescher N, Nell TA, Dominick L, Midgley N, Deshpande G, Page MJ, Pretorius E, Woudberg NJ, Lecour S, Glashoff RH, Essop MF (2019) HIV and cardiovascular diseases risk: exploring the interplay between T-cell activation, coagulation, monocyte subsets, and lipid subclass alterations. Am J Physiol - Heart Circ Physiol 316:H1146–H1157
Valverde-Villegas JM, Matte MCC, De MRM, Chies JAB (2015) New Insights about Treg and Th17 Cells in HIV infection and disease progression. J Immunol Res 2015:1–14
Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF (2003) Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest 112:1437–1443
Wan YY, Flavell RA (2007) ‘Yin–Yang’ functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol Rev 220:199–213
Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D (2009) Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci 106:13439–13444
Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J (2005) Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A 102:4091–4096
Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE (2008) Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis 67:1037–1040
Acknowledgments
This work was supported by South African Medical Research Council and Stellenbosch University (to M. F. Essop). We acknowledge Nazma Mansoor and Rozanne Adam for excellent assistance with flow cytometry.
Author information
Authors and Affiliations
Contributions
ET, RHG, and MFE conceived and designed research. ET, DEJ and LD performed experiments. ET, DEJ, LD, RHG, and MFE analyzed data. ET, DEJ, LD, RHG, and MFE interpreted results of experiments. ET and DEJ prepared figures. ET, DEJ, RHG, and MFE drafted manuscript. ET, DEJ, RHG, and MFE edited and revised manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
Ethical clearance was obtained from the Human Research Ethics Committee of Stellenbosch University (N12/12/086) and the Department of Health in the Western Cape, South Africa (reference number: RP090/2013). Prior to the study, we informed participants (attending the Worcester Community Day Center) about the various study procedures and subsequently obtained written consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teer, E., Joseph, D.E., Dominick, L. et al. Expansion of GARP-Expressing CD4+CD25−FoxP3+ T Cells and SATB1 Association with Activation and Coagulation in Immune Compromised HIV-1-Infected Individuals in South Africa. Virol. Sin. 36, 1133–1143 (2021). https://doi.org/10.1007/s12250-021-00386-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-021-00386-8