Abstract
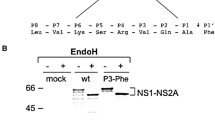
Flaviviral NS2B is a required cofactor for NS3 serine protease activity and plays an important role in promoting functional NS2B-NS3 protease configuration and maintaining critical interactions with protease catalysis substrates. The residues D80DDG in West Nile virus (WNV) NS2B are important for protease activity. To investigate the effects of D80DDG in NS2B on protease activity and viral replication, the negatively charged region D80DD and the conserved residue G83 of NS2B were mutated (D80DD/E80EE, D80DD/K80KK, D80DD/A80AA, G83F, G83S, G83D, G83K, and G83A), and NS3 D75A was designated as the negative control. The effects of the mutations on NS2B-NS3 activity, viral translation, and viral RNA replication were analyzed using kinetic analysis of site-directed enzymes and a transient replicon assay. All substitutions resulted in significantly decreased enzyme activity and blocked RNA replication. The negative charge of D80DD is not important for maintaining NS2B function, but side chain changes in G83 have dramatic effects on protease activity and RNA replication. These results demonstrate that NS2B is important for viral replication and that D80DD and G83 substitutions prevent replication; they will be useful for understanding the relationship between NS2B and NS3.
Article PDF
Similar content being viewed by others
References
Chappell K J, Nall T A, Stoermer M J, Fang N X, Tyndall J D, Fairlie D P, Young P R. 2005. Site-directed Mutagenesis and Kinetic Studies of the West Nile Virus NS3 Protease Identify Key Enzyme-Substrate Interactions. J Biol Chem, 280(4):2896–2903.
Chappell K J, Stoermer M J, Fairlie D P, Young P R. 2008. Mutagenesis of the West Nile virus NS2B cofactor domain reveals two regions essential for protease activity. J Gen Virol, 89(Pt4):1010–1014.
Chambers T J, Nestorowicz A, Amberg S M, Rice C M. 1993. Mutagenesis of the yellow fever virus NS2B protein: effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J Virol, 67(11):6797–6807.
Ciota A T, Lovelace A O, Ngo K A, Le A N, Maffei J G, Franke M A, Payne A F, Jones S A, Kauffman E B, Kramer L D. 2007. Cell-specific adaptation of two flaviviruses following serial passage in mosquito cell culture. Virology, 357(2):165–174.
D’Arcy A, Chaillet M, Schiering N, Villard F, Lim S P, Lefeuvre P, Erbel P. 2006. Purification and crystallization of dengue and West Nile virus NS2B-NS3 complexes. Acta Crystallogr Sect F Struct Biol Cryst Commun, 62(Pt2):157–162.
Droll D A, Krishna Murthy H M, Chambers T J. 2000. Yellow fever virus NS2B-NS3 protease: charged-to-alanine mutagenesis and deletion analysis define regions important for protease complex formation and function. Virology, 275(2):335–347.
Erbel P, Schiering N, D’Arcy A, Renatus M, Kroemer M, Lim S P, Yin Z, Keller T H, Vasudevan S G, Hommel U. 2006. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol, 13(4):372–373.
Falgout B, Miller R H, Lai C J. 1993. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J Virol, 67(4): 2034–2042.
Falgout B, Pethel M, Zhang Y M, Lai C J. 1991. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol, 65(5):2467–2475.
Jia F, Zou G, Fan J J, Yuan Z M. 2010. Identification of palmatine as an inhibitor of West Nile virus. Arch Virol, 155(8): 1325–1329.
Johnston P A, Phillips J, Shun T Y, Shinde S, Lazo J S, Huryn D M, Myers M C, Ratnikov B I, Smith J W, Su Y, Dahl R, Cosford N D, Shiryaev S A, Strongin A Y. 2007. HTS Identifies Novel and Specific Uncompetitive Inhibitors of the Two-Component NS2B-NS3 Proteinase of West Nile Virus. Assay Drug Dev Technol, 5(6):737–750.
Leung D, Schroder K, White H, Fang N X, Stoermer M J, Abbenante G, Martin J L, Young P R, Fairlie D P. 2001. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J Biol Chem, 276(49):45762–45771.
Lin C W, Huang H D, Shiu S Y, Chen W J, Tsai M H, Huang S H, Wan L, Lin Y J. 2007. Functional determinants of NS2B for activation of Japanese encephalitis virus NS3 protease. Virus Res, 127(1):88–94.
Lo M K, Tilgner M, Bernard K A, Shi P Y. 2003. Functional analysis of mosquito-borne flavivirus conserved sequence elements within 3’ untranslated region of West Nile virus by use of a reporting replicon that differentiates between viral translation and RNA replication. J Virol, 77(18):10004–10014.
Loughlin W A, Tyndall J D, Glenn M P, Fairlie D P. 2004. Beta-strand mimetics. Chem Rev, 104(12):6085–6117.
Mueller N H, Yon C, Ganesh V K, Padmanabhan R. 2007. Characterization of the West Nile virus protease substrate specificity and inhibitors. Int J Biochem Cell Biol, 39(3):606–614.
Mukhopadhyay S, Kuhn R J, Rossmann M G. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol, 3(1):13–22.
Nall T A, Chappell K J, Stoermer M J, Fang N X, Tyndall J D, Young P R, Fairlie D P. 2004. Enzymatic Characterization and Homology Model of a Catalytically Active Recombinant West Nile Virus NS3 Protease. J Biol Chem, 279(47):48535–48542.
Radichev I, Shiryaev S A, Aleshin A E, Ratnikov B I, Smith J W, Liddington R C, Strongin A Y. 2008. Structure-based mutagenesis identifies important novel determinants of the NS2B cofactor of the West Nile virus two-component NS2B-NS3 proteinase. J Gen Virol, 89(Pt3):636–641.
Ryan M D, Monaghan S, Flint M. 1998. Virus-encoded proteinases of the Flaviviridae. J Gen Virol. 79(Pt5):947–959.
Shiryaev S A, Ratnikov B I, Chekanov A V, Sikora S, Rozanov D V, Godzik A, Wang J, Smith J W, Huang Z, Lindberg I, Samuel M A, Diamond M S, Strongin A Y. 2006. Cleavage targets and the D-arginine-based inhibitors of the West Nile virus NS3 processing proteinase. Biochem J, 393(Pt2):503–511.
Stadler K, Allison S L, Schalich J, Heinz F X. 1997. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol, 71(11):8475–8481.
Thomas G. 2002. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol, 3(10):753–766.
Tilgner M, Shi P Y. 2004. Structure and function of the 3′ terminal six nucleotides of the west nile virus genome in viral replication. J Virol, 78(15):8159–8171.
Tyndall J D, Nall T, Fairlie D P. 2005. Proteases universally recognize beta strands in their active sites. Chem Rev, 105(3):973–999.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation items: Supported by Important National Science & Technology Specific Projects (2012ZX10004403, 2012ZX10004219).
Rights and permissions
About this article
Cite this article
Jia, F., Fan, J., Zhang, B. et al. Mutagenesis of D80-82 and G83 residues in West Nile Virus NS2B: Effects on NS2B-NS3 activity and viral replication. Virol. Sin. 28, 16–23 (2013). https://doi.org/10.1007/s12250-013-3276-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-013-3276-y