Abstract
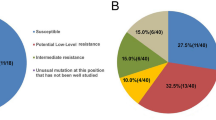
This study aimed to evaluate emerging trends of drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) among 290 former blood donor HIV-1 infected patients in Hubei, China, from 2004 to 2006, all of whom had received anti-HIV-1 therapy. The presence of NRTI- and NNRTI-associated mutations were established by sequencing; genotypic and predicted phenotypic drug resistance were evaluated using HIVdb Program version 5.0.1 (http://hivdb.stanford.edu/pages/algs/HIVdb.html). Genotypic drug resistance analysis showed significant increases in percentages of patients carrying HIV-1 strains with M41L, T215Y/F, D67N, K103N, G190A/S, Y181C/F or L210W mutations. Of the variants’ predicted phenotypic drug resistance, highly significant increases were detected in percentages of patients carrying HIV-1 with high resistance to zidovudine (AZT) or stavudine (D4T) in NRTIs, and to delavirdine (DLV), efavirenz (EFV) or nevirapine (NVP) in NNRTIs; intermediate resistance to abacavir (ABC), AZT, D4T, didanosine (DDI) or tenofovir disoproxil fumarate (TDF) in NRTIs, and to etravirine (ETR) in NNRTIs; and low and potential low resistance to lamivudine (3TC), ABC, emtricitabine (FTC) or TDF in NRTIs, and to ETR in NNRTIs.
Similar content being viewed by others
References
Antinori A, Zaccarelli M, Cingolani A, et al. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retrov, 18: 835–838.
Calvez V, Costagliola D, Descamps D, et al. 2002. Impact of stavudine phenotype and thymidine analogues mutations on viral response to stavudine plus lamivudine in ALTIS 2 ANRS trial. Antivir Ther, 7: 211–218.
De Clercq E. 2009. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Ag, 33: 307–320.
Gingeras T, Mamtora G, Shen N, et al. 1996. Genetic analysis of HIV-1 plasma using high density oligonucleotide arrays and dideoxynucleotide sequencing. Antivral Therapy, 1(Suppl.1): 42.
Hertogs K, de Bethune M P, Miller V, et al. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Ch, 42: 269–276.
Japour A J, Mayers D L, Johnson V A, et al. 1993. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Ch, 37: 1095–1101.
Johnson V A, Brun-Vezinet F, Clotet B, et al. 2008. Update of the drug resistance mutations in HIV-1: Spring 2008. Top HIV Med, 16: 62–68.
Kantor R, Machekano R, Gonzales M J, et al. 2001. Human Immunodeficiency Virus Reverse Transcriptase and Protease Sequence Database: an expanded data model integrating natural language text and sequence analysis programs. Nucleic Acids Research, 29: 296–299.
Lecossier D, Shulman N S, Morand-Joubert L, et al. 2005. Detection of minority populations of HIV-1 expressing the K103N resistance mutation in patients failing nevirapine. J Acq Immune Defic Syndr, 38: 37–42.
Liu P, Xiang K, Tang H, et al. 2008. Molecular epidemiology of human immunodeficiency virus type 1 and hepatitis C virus in former blood donors in central China. AIDS Res Hum Retrov, 24: 1–6.
Marcelin A G, Flandre P, Pavie J, et al. 2005. Clinically relevant genotype interpretation of resistance to didanosine. Antimicrob Agents Ch, 49: 1739–1744.
Rhee S Y, Gonzales M J, Kantor R, et al. 2003. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Research, 31: 298–303.
Shafer R W, Winters M A, Palmer S, et al. 1998. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from heavily treated patients. Ann Intern Med, 128: 906–911.
Wensing A M, van de Vijver D A, Angarano G, et al. 2005. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis, 192: 958–966.
Yerly S, Kaiser L, Race E, et al. 1999. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet, 354: 729–733.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation items: The Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period of China (2008ZX10001-002), and the Major Science and Technology Innovation Cross Project of the Chinese Academy of Sciences (KSCX1-YW-10).
Rights and permissions
About this article
Cite this article
Gong, J., Wang, Xq., Tong, X. et al. Emerging trends of drug-resistant HIV-1 among drug-treated patients in former blood donors in Hubei, China: a three-year surveillance from 2004 to 2006. Virol. Sin. 26, 386–392 (2011). https://doi.org/10.1007/s12250-011-3210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-011-3210-0