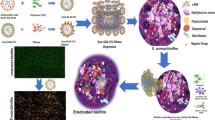
Abstract
Purpose
This study developed a new formulation for silver sulfadiazine (SSD) nanocrystal-based hydrogels for in vitro antimicrobial activity.
Methods
SSD nanocrystals were prepared by using a wet-mill apparatus; effects of polymers, surfactants and lipid-based carriers were investigated. The gel-forming chemicals were subsequently dispersed in the SSD nanocrystal nanosuspensions, resulting in homogeneous hydrogels. The antibacterial activities of new formulations were tested in-vitro.
Results
The final SSD nanocrystal formulation (less than 300 nm, PDI: 0.300) containing glyceryl monostearate (GMS) or lecithin (Lec), combining with hydrophilic polymers (hydroxypropyl methyl cellulose and polyvinyl alcohol). The artificial neural network was utilized to confirm the effects of lipid ingredients in wet-milling process. Hydroxyethyl cellulose was chosen to formulate the hydrogel which formed the white, smooth, homogeneous, and stable hydrogel after 4 weeks at the room condition. The hydrogel also presented higher and more sustained drug release using Franz’s diffusion cells as compared with reference marketed drug and control hydrogels. The efficacy of antibacterial activity shown on the biofilm demonstrated effect of particle size, lipid carriers, and probably interaction between SSD with biofilm membrane.
Conclusion
These findings implied a potential application of SSD nanocrystal-lipid carrier-based hydrogels in clinical practice.






Similar content being viewed by others
References
Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatology. 2007;127(3):514–25.
Miller AC, Rashid RM, Falzon L, Elamin EM, Zehtabchi S. Silver sulfadiazine for the treatment of partial-thickness burns and venous stasis ulcers. J Am Acad Dermatol. 2012;66(5):e159–65.
Hoffmann S. Silver sulfadiazine: an antibacterial agent for topical use in burns. Scand J Plast Reconstr Surg. 1984;18(1):119–26.
Ueda Y, Miyazaki M, Mashima K, Takagi S, Hara S, Kamimura H et al. The effects of silver sulfadiazine on methicillin-resistant Staphylococcus aureus biofilms. 2020;8(10):1551.
Kumar PM, Ghosh A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur J Pharm Sci. 2017;96:243–54.
Naser NA, Alasedi KM, Khan ZA. New approach for determination of sulfadiazine in pharmaceutical preparations using 4 (4-sulphophenylazo) pyrogallol: kinetic spectrophotometric method. Spectrochim Acta Part A Mol Biomol Spectrosc. 2018;201:267–80.
Nesbitt RU Jr., Sandmann BJ. Solubility studies of silver sulfadiazine. J Pharm Sci. 1977;66(4):519–22.
Pharmacopoeia J. Japanese Pharmacopoeia 17: Official Monographs Sulfadiazine Silve. 2016.
Costerton JW, Stewart PS, Greenberg EPJ. Bacterial biofilms: a common cause of persistent infections. 1999;284(5418):1318–22.
Jahangir MA, Imam SS, Muheem A, Chettupalli A, Al-Abbasi FA, Nadeem MS, et al. Nanocrystals: characterization overview, applications in drug delivery, and their toxicity concerns. J Pharm Innov. 2022;17(1):237–48.
Venkataraman M, Nagarsenker M. Silver sulfadiazine nanosystems for burn therapy. AAPS PharmSciTech. 2013;14(1):254–64.
Gao L, Gan H, Meng Z, Gu R, Wu Z, Zhu X, et al. Evaluation of genipin-crosslinked chitosan hydrogels as a potential carrier for silver sulfadiazine nanocrystals. Colloid Surf B: Biointerfaces. 2016;148:343–53.
Liu X, Gan H, Hu C, Sun W, Zhu X, Meng Z et al. Silver sulfadiazine nanosuspension-loaded thermosensitive hydrogel as a topical antibacterial agent. Int J Nanomed. 2019:289–300.
Mastiholimath VS, Valerie CTW, Shrishailappa V, Mannur PMD, Gadad AP, Khanal P. Formulation and evaluation of solid lipid nanoparticle containing silver sulfadiazine for second and third degree burn wounds and its suitable analytical method development and validation. Indian J Pharm Educ Res. 2020;54:31–45.
Malamatari M, Taylor KM, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discovery Today. 2018;23(3):534–47.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Nguyen TK, Peyrusson F, Siala W, Pham NH, Nguyen HA, Tulkens PM, et al. Activity of Moxifloxacin Against Biofilms formed by clinical isolates of Staphylococcus aureus Differing by their resistant or Persister Character to fluoroquinolones. Front Microbiol. 2021;12:785573.
Tuomela A, Hirvonen J, Peltonen LJP. Stabilizing agents for drug nanocrystals: effect on bioavailability. 2016;8(2):16.
Sargam Y, Wang K, Tsyrenova A, Liu F, Jiang S. Effects of anionic and nonionic surfactants on the dispersion and stability of nanoSiO2 in aqueous and cement pore solutions. Cem Concr Res. 2021;144:106417.
Attama AA, Momoh MA, Builders PF. Lipid nanoparticulate drug delivery systems: a revolution in dosage form design and development. Recent Adv Novel drug Carrier Syst. 2012;5:107–40.
Tran BN, Tran HT, Le GT, Tran HP, Le KN, Do HH et al. Solidifying Fenofibrate Nanocrystal Suspension: A Scalable Approach via Granulation Method. 2023;2023.
Tran BN, Nguyen HT, Kim JO, Yong CS, Nguyen, CNJAopr. Combination of a chemopreventive agent and paclitaxel in CD44-targeted hybrid nanoparticles for breast cancer treatment. 2017;40:1420–32.
Bult A, Plug CM. Silver sulfadiazine. Analytical profiles of Drug Substances. Volume 13. Elsevier; 1984. pp. 553–71.
Sheskey PJHB, Moss GP, Goldfarb DJ. Handbook of pharmaceutical excipients: Edition 9. Pharmaceutical; 2020.
Iwai I, Han H, Hollander Ld, Svensson S, Öfverstedt L-G, Anwar J, et al. The human skin barrier is Organized as stacked bilayers of fully extended ceramides with Cholesterol Molecules Associated with the Ceramide Sphingoid Moiety. J Invest Dermatology. 2012;132(9):2215–25.
Dannert C, Stokke BT, Dias RS. Nanoparticle-hydrogel composites: from molecular interactions to macroscopic behavior. 2019;11(2):275.
Tran BN, Tran KL, Nguyen TT, Bui LT, Nguyen CN. A Novel Alginate Film based on Nanocoating Approach for enteric-release tablets. AAPS PharmSciTech. 2023;24(4):99.
Nguyen TK, Argudín MA, Deplano A, Nhung PH, Nguyen HA, Tulkens PM et al. Antibiotic resistance, biofilm formation, and intracellular survival as possible determinants of persistent or recurrent infections by staphylococcus aureus in a Vietnamese tertiary hospital: focus on bacterial response to moxifloxacin. 2020;26(6):537–44.
Siala W, Kucharíková S, Braem A, Vleugels J, Tulkens PM, Mingeot-Leclercq M-P, et al. The antifungal caspofungin increases fluoroquinolone activity against Staphylococcus aureus biofilms by inhibiting N-acetylglucosamine transferase. Nat Commun. 2016;7(1):13286.
Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo, JJRim. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. 2005;156(4):506–14.
Mizdal CR, Stefanello ST, da Costa Flores V, Agertt VA, Bonez PC, Rossi GG et al. The antibacterial and anti-biofilm activity of gold-complexed sulfonamides against methicillin-resistant Staphylococcus aureus. 2018;123:440–8.
Vaze N, Demokritou P. Using engineered water nanostructures (EWNS) for wound disinfection: case study of Acinetobacter baumannii inactivation on skin and the inhibition of biofilm formation. Nanomed Nanotechnol Biol Med. 2022;42:102537.
Siqueira FS, Alves CFS, Machado AK, Siqueira JD, Santos Td, Mizdal CR et al. Molecular docking, quorum quenching effect, antibiofilm activity and safety profile of silver-complexed sulfonamide on Pseudomonas aeruginosa. 2021;37(5):555–71.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luong, A.Q., Pham, H.TT., Tran, B.N. et al. Silver Sulfadiazine Nanocrystal-Based Hydrogels: The Impact of Lipid Components on In-vitro and Ex-vivo Release, Bacterial Biofilm Permeability, and In-vitro Antibacterial Activity. J Pharm Innov 19, 24 (2024). https://doi.org/10.1007/s12247-024-09834-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09834-w