Abstract
Purpose
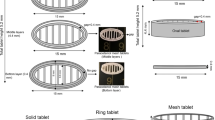
Drug release from hypromellose-based tablets involves the formation of characteristic dry cores surrounded by outer gel layers in aqueous media. The aim of this study was to investigate the effect of perforation sizes on the dissolution of repaglinide from three-dimensionally (3D) printed tablets with two viscosity grades of hypromellose as rate-controlling polymer.
Methods
Printing pastes of appropriate consistency were developed and fed into a bioplotter cartridge to extrude strands/filaments. Tablets were printed in a crisscross pattern with 1.0, 1.3, and 1.6 mm of inter-strand distances. Printed tablets were characterized and repaglinide dissolution data were evaluated mathematically.
Results
Scanning electron microscopy images confirmed the sealing of the defect-free strands. Differential scanning calorimetry studies indicated potential interaction of ingredients above 150 °C which suggested that the formulation, printing, and drying of tablets should be performed at room temperature. All of the tablets possessed acceptable size, shape, and mechanical strength. Repaglinide dissolution data were evaluated by similarity factor (f2), dissolution efficiency, and two-way analysis of variance (ANOVA) which revealed that drug release rate and extent could be gradually increased by increasing the perforation sizes inside the tablets. Korsmeyer-Peppas modeling of dissolution data showed that the drug release rate approached zero-order kinetics and the release mechanism was Super Case II. This mechanism implies that an outer layer of HPMC gel limits the swelling of the characteristic HPMC tablet cores.
Conclusion
The cores in HPMC tablets usually remain dry for long time in aqueous media. Higher perforation sizes enhanced repaglinide dissolution rate from such tablets. This led us to conclude that different perforation sizes in the tablets must have caused differences in the structural consistency of the core. Perforation sizes played the dominant role over HPMC viscosity grades in determining repaglinide release.









Similar content being viewed by others
References
Ngo TD, Kashani A, Imbalzano G, Nguyen KT, Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos B Eng. 2018;143:172–96.
Zhang B, Luo Y, Ma L, Gao L, Li Y, Xue Q, et al. 3D bioprinting: an emerging technology full of opportunities and challenges. Bio-Design and Manufacturing. 2018;1(1):2–13.
Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm Res. 2018;35(9):1–22.
Levetiracetam Approval Package, Application number: 207958Orig1s000. In: Drugs@FDA: FDA-approved drugs. Center for Drug Evaluation and Research, Food and Drug Administration, USA. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf. Accessed 15 Jan 2022
Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–63.
Khaled SA, Alexander MR, Irvine DJ, Wildman RD, Wallace MJ, Sharpe S, et al. Extrusion 3D printing of paracetamol tablets from a single formulation with tunable release profiles through control of tablet geometry. AAPS PharmSciTech. 2018;19(8):3403–13.
Martinez PR, Goyanes A, Basit AW, Gaisford S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech. 2018;19(8):3355–61.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–50.
Pereira BC, Isreb A, Isreb M, Forbes RT, Oga EF, Alhnan MA (2020) Computer‐aided drug design: additive manufacturing of a point‐of‐care “Polypill:” Fabrication of Concept Capsules of Complex Geometry with Bespoke Release against Cardiovascular Disease (Adv. Healthcare Mater. 13/2020). Adv Healthcare Mater 9(13):2070042
Maroni A, Melocchi A, Parietti F, Foppoli A, Zema L, Gazzaniga A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J Control Release. 2017;268:10–8.
Jamróz W, Kurek M, Łyszczarz E, Szafraniec J, Knapik-Kowalczuk J, Syrek K, et al. 3D printed orodispersible films with Aripiprazole. Int J Pharm. 2017;533(2):413–20.
Holländer J, Genina N, Jukarainen H, Khajeheian M, Rosling A, Mäkilä E, et al. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J Pharm Sci. 2016;105(9):2665–76.
Fu J, Yu X, Jin Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int J Pharm. 2018;539(1–2):75–82.
Meyer JM, Ginsburg GS. The path to personalized medicine. Curr Opin Chem Biol. 2002;6(4):434–8.
Foster SL, Petrie SR, Mischoulon D, Fava M. Personalized Medicine. In: Shapero BG, Mischoulon D, Cusin C, editors. The Massachusetts General Hospital Guide to Depression. Switzerland: Humana Press Cham; 2019. p. 109–21.
Milner Z, Akhondi H (2022) Repaglinide. In: StatPearls [Internet] Publishing. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK559305/. Accessed 2 May 2022
Mandić Z, Gabelica V. Ionization, lipophilicity and solubility properties of repaglinide. J Pharm Biomed Anal. 2006;41(3):866–71.
Pandey SS, Patel MA, Desai DT, Patel HP, Gupta AR, Joshi SV, et al. Bioavailability enhancement of repaglinide from transdermally applied nanostructured lipid carrier gel: optimization, in vitro and in vivo studies. Journal of Drug Delivery Science and Technology. 2020;57: 101731.
Yin LF, Huang SJ, Zhu CL, Zhang SH, Zhang Q, Chen XJ, et al. In vitro and in vivo studies on a novel solid dispersion of repaglinide using polyvinylpyrrolidone as the carrier. Drug Dev Ind Pharm. 2012;38(11):1371–80.
Nanjwade BK, Varia PJ, Kadam VT, Srichana T, Kamble MS. Development and evaluation of nanoemulsion of repaglinide. Nanotechnol Nanomed. 2013;1(2):1–8.
Zhu Z, Yang T, Zhao Y, Gao N, Leng D, Ding P. A simple method to improve the dissolution of repaglinide and exploration of its mechanism. Asian J Pharm Sci. 2014;9(4):218–25.
Li CL, Martini LG, Ford JL, Roberts M. The use of hypromellose in oral drug delivery. J Pharm Pharmacol. 2005;57(5):533–46.
Siyawamwaya M, du Toit LC, Kumar P, Choonara YE, Kondiah PP, Pillay V. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur J Pharm Biopharm. 2019;138:99–110.
He W, Wu M, Huang S, Yin L. Matrix tablets for sustained release of repaglinide: preparation, pharmacokinetics and hypoglycemic activity in beagle dogs. Int J Pharm. 2015;478(1):297–307.
Validation of analytical procedures: text and methodology, ICH Harmonized Tripartite Guideline Q2 (R1). In: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf. Accessed 20 Jan 2022
Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461(1–2):105–11.
USP (2012) TABLET FRIABILITY 〈1216〉. In: USP-NF Rockville, MD: USP; May 1, 2012
Moore JW. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–75.
Xie F, Ji S, Cheng Z. In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example. Eur J Pharm Sci. 2015;66:163–72.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27(1):48–9.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48:139–57.
Bruschi ML. Strategies to modify the drug release from pharmaceutical systems. Cambridge: Woodhead Publishing; 2015. p. 65–8.
Buszewski B, Šebeková K. Perturbation of baseline in HPLC analysis as the consequence of sample injection. J High Resolut Chromatogr. 1988;11(8):598–600. https://doi.org/10.1002/jhrc.1240110811.
Paxton N, Smolan W, Böck T, Melchels F, Groll J, Jungst T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication. 2017;9(4): 044107.
Zidan A, Alayoubi A, Coburn J, Asfari S, Ghammraoui B, Cruz CN, et al. Extrudability analysis of drug loaded pastes for 3D printing of modified release tablets. Int J Pharm. 2019;554:292–301.
Scoutaris N, Ross SA, Douroumis D. 3D printed “Starmix” drug loaded dosage forms for paediatric applications. Pharm Res. 2018;35(2):1–1.
Bozdağ-Pehlivan S, Subaşi B, Vural I, Unlü N, Capan Y. Evaluation of drug-excipient interaction in the formulation of celecoxib tablets. Acta Pol Pharm. 2011;68(3):423–33.
Rojek B, Wesolowski M. DSC supported by factor analysis as a reliable tool for compatibility study in pharmaceutical mixtures. J Therm Anal Calorim. 2019;138:4531–9. https://doi.org/10.1007/s10973-019-08223-7.
Chadha R, Bhandari S. Drug-excipient compatibility screening–role of thermoanalytical and spectroscopic techniques. J Pharm Biomed Anal. 2014;87:82–97. https://doi.org/10.1016/j.jpba.2013.06.016.
Pani NR, Nath LK, Acharya S. Compatibility studies of nateglinide with excipients in immediate release tablets. Acta Pharm. 2011;61(2):237–47. https://doi.org/10.2478/v10007-011-0016-4.
Rogers TL, Penz FK, Zelenik JA. Hypromellose. In: Sheskey PJ, Cook WG, Cable CG, editors. Handbook of Pharmaceutical Excipients. London: Pharmaceutical Press; 2017. p. 468–72.
Penz FK, Zelenik JA. Lactose, Anhydrous. In: Sheskey PJ, Cook WG, Cable CG, editors. Handbook of Pharmaceutical Excipients. London: Pharmaceutical Press; 2017. p. 506–9.
Chen D, Xu XY, Li R, Zang GA, Zhang Y, Wang MR, et al. Preparation and in vitro evaluation of FDM 3D-printed ellipsoid-shaped gastric floating tablets with low infill percentages. AAPS PharmSciTech. 2020;21(1):1–3.
Pham AT, Lee PI. Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm Res. 1994;11(10):1379–84.
Siepmann J, Podual K, Sriwongjanya M, Peppas NA, Bodmeier R. A new model describing the swelling and drug release kinetics from hydroxypropyl methylcellulose tablets. J Pharm Sci. 1999;88(1):65–72.
Colombo P, Conte U, Gazzaniga A, Maggi L, Sangalli ME, Peppas NA, et al. Drug release modulation by physical restrictions of matrix swelling. Int J Pharm. 1990;63(1):43–8.
Shah VP, Tsong Y, Sathe P, Williams RL. Dissolution profile comparison using similarity factor, f2. Dissolution Technol. 1999;6(3):15.
Acknowledgements
This work belongs to the thesis, “Three-dimensional Printing of Antidiabetic Tablets,” completed in the program of “Masters in Drug and Cosmetics Production Technologies,” Institute of Health Sciences, Yeditepe University, Istanbul, with Ahmed Wadi as the researcher and Dr. Muhammed Abdur Rauf as the adviser. We sincerely thank Prof. Dr. Gamze Torun Köse from the Department of Genetics and Bioengineering, Yeditepe University, for providing access to the EnvisionTEC 3D-Biopltter. We also thank Prof. Dr. Çetin Taş from the Department of Pharmaceutical Technology of the same university.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ahmed Mohammed Wadi. Organization of material supply and workflow, drafting of the work and revising it critically for important intellectual content were performed by Dr Muhammed Abdur Rauf. The first draft of the manuscript was written by Dr Muhammed Abdur Rauf and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wadi, A., Rauf, M.A. Three-Dimensional Printing of Repaglinide Tablets: Effect of Perforations on Hypromellose-Based Drug Release. J Pharm Innov 18, 837–850 (2023). https://doi.org/10.1007/s12247-022-09684-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-022-09684-4