Abstract
Purpose
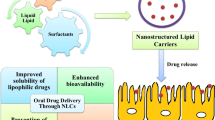
Lumefantrine (LF) is an antimalarial agent with low water solubility and inconsistent oral bioavailability (4–11%), which limits its therapeutic potential. Hence, this research aimed to design nanostructured lipid carriers (NLCs) for LF to improve its oral administration.
Methods
LF-NLCs were formulated using the hot emulsification-probe sonication method. Solubility and molecular interactions studies were performed for the selection of appropriate formulation excipients. Various critical formulation and process variables were optimized during this process. The optimized LF-NLC was then evaluated for various physicochemical properties. Ex vivo gut permeation was done to analyze LF-NLC permeation across the gut, and in vitro drug release was performed to understand its release behavior.
Results
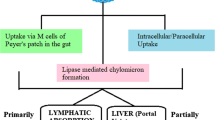
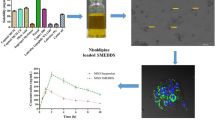
Particle size, polydispersity index (PDI), zeta potential, and % encapsulation efficiency of the optimized formulation were found to be 78.85 ± 2.55 nm, 0.255 ± 0.03, -8.25 ± 2.29 mV, and 74.32 ± 1.63%, respectively. Transmission electron microscopy revealed the particulate nature and spherical shape of LF-NLCs. The loss of sharp endothermic peak in DSC and reduced crystallinity in XRD indicated the amorphization of encapsulated drug. The in vitro drug release study showed sustained release of LF from NLC. Ex vivo gut permeation exhibited an almost 3.5-fold increase in permeation of NLCs over plain drug suspension.
Conclusion
According to the above findings, NLC could be a promising approach for the oral delivery of LF and drugs with similar properties.








Similar content being viewed by others
References
Gahoi S, Jain GK, Tripathi R, Pandey SK, Anwar M, Warsi MH, et al. Enhanced antimalarial activity of lumefantrine nanopowder prepared by wet-milling DYNO MILL technique. Colloids Surfaces B Biointerfaces. 2012;95:16–22.
Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43–8.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm Hindawi Limited. 2012;2012:1–10.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm Elsevier. 2004;58:265–78.
Patel K, Sarma V, Vavia P. Design and evaluation of Lumefantrine – oleic acid self nanoemulsifying ionic complex for enhanced dissolution. DARU J Pharm Sci. 2013;21:27.
Lumefantrine: uses, interactions, mechanism of action | DrugBank Online [Internet]. [cited 2021 Sep 11]. Available from: https://go.drugbank.com/drugs/DB06708.
Wernsdorfer W, Landgraf B, Kilimali V, Wernsdorfer G. Activity of benflumetol and its enantiomers in fresh isolates of Plasmodium falciparum from East Africa. Acta Trop. 1998;70:9–15.
Murambiwa P, Masola B, Govender T, Mukaratirwa S, Musabayane CT. Anti-malarial drug formulations and novel delivery systems: a review. Acta Trop. 2011;118:71–9.
van Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, et al. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug resistant falciparum malaria. Antimicrob Agents Chemother. American Society for Microbiology Journals. 1998;42:135–9.
Wahajuddin, Raju KSR, Singh SP, Taneja I. Investigation of the functional role of P-glycoprotein in limiting the oral bioavailability of lumefantrine. Antimicrob Agents Chemother. American Society for Microbiology (ASM). 2014;58:489–94.
Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704.
Patil S, Suryavanshi S, Pathak S, Sharma S, Patravale V. Evaluation of novel lipid based formulation of β-artemether and lumefantrine in murine malaria model. Int J Pharm. 2013;455:229–34.
Fule R, Meer T, Sav A, Amin P. Solubility and dissolution rate enhancement of lumefantrine using hot melt extrusion technology with physicochemical characterisation. J Pharm Investig. 2013;43:305–21.
Shah R, Soni T, Shah U, Suhagia BN, Patel MN, Patel T, et al. Formulation development and characterization of lumefantrine nanosuspension for enhanced antimalarial activity. J Biomater Sci Polym Ed. Taylor & Francis. 2021;32:833–57.
Trasi NS, Bhujbal SV, Zemlyanov DY, Zhou QT, Taylor LS. Physical stability and release properties of lumefantrine amorphous solid dispersion granules prepared by a simple solvent evaporation approach. Int J Pharm: X. Elsevier. 2020;2:100052.
Hiew TN, Zemlyanov DY, Taylor LS. Balancing solid-state stability and dissolution performance of lumefantrine amorphous solid dispersions: the role of polymer choice and drug–polymer interactions. Mol Pharm. American Chemical Society. 2021;acs.molpharmaceut.1c00481.
Tay E, Nguyen T-H, Ford L, Williams HD, Benameur H, Scammells PJ, et al. Ionic liquid forms of the antimalarial lumefantrine in combination with LFCS type IIIB lipid-based formulations preferentially increase lipid solubility, in vitro solubilization behavior and in vivo exposure. Pharmaceutics. Multidisciplinary Digital Publishing Institute. 2019;12:17.
Akpa PA, Ugwuoke JA, Attama AA, Ugwu CN, Ezeibe EN, Momoh MA, et al. Improved antimalarial activity of caprol-based nanostructured lipid carriers encapsulating artemether-lumefantrine for oral administration. Afr Health Sci. Makerere University, Medical School. 2020;20:1679–97.
Pawar S, Shende P. Dual drug delivery of cyclodextrin cross-linked artemether and lumefantrine nanosponges for synergistic action using 23 full factorial designs. Colloids Surfaces A Physicochem Eng Asp. Elsevier. 2020;602:125049.
Byakika-Kibwika P, Lamorde M, Mayanja-Kizza H, Khoo S, Merry C, Van geertruyden J-P. Artemether-lumefantrine combination therapy for treatment of uncomplicated malaria: the potential for complex interactions with antiretroviral drugs in HIV-infected individuals. Malar Res Treat. Hindawi. 2011;2011:1–5.
Kumbhar DD, Pokharkar VB. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: physicochemical investigations. Colloids Surfaces A Physicochem Eng Asp. 2013;416:32–42.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55.
Shevalkar G, Vavia P. Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. J Drug Deliv Sci Technol. Elsevier. 2019;53:101211.
Aditya NP, Patankar S, Madhusudhan B, Murthy RSR, Souto EB. Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur J Pharm Sci. 2010;40:448–55.
Parashar D, Aditya NP, Murthy RSR. Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: physicochemical characterization and in vivo antimalarial activity. Drug Deliv. 2016;23:123–9.
Neupane YR, Sabir MD, Ahmad N, Ali M, Kohli K. Lipid drug conjugate nanoparticle as a novel lipid nanocarrier for the oral delivery of decitabine: ex vivo gut permeation studies. Nanotechnology. 2013;24:1–11.
Neupane YR, Srivastava M, Ahmad N, Kumar N, Bhatnagar A, Kohli K. Lipid based nanocarrier system for the potential oral delivery of decitabine: formulation design, characterization, ex vivo, and in vivo assessment. Int J Pharm. Elsevier BV. 2014;477:601–12.
Perkins EG. Nutritional and chemical changes occurring in heated fats: A review. Food Technol. 1960;14:508–14.
Schultz HW. Symposium on foods: lipids and their oxidation. Symp Foods Lipids Their Oxid 1961 Oregon State Univ. Avi Pub Co.; Westport, Conn., Avi Pub Co., 1962;1962.
Stella V, Haslam J, Yata N, Okada H, Lindenbaum S, Higuchi T. Enhancement of bioavailability of a hydrophobic amine antimalarial by formulation with oleic acid in a soft gelatin capsule. J Pharm Sci. 1978;67:1375–7.
Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta - Biomembr Elsevier. 2009;1788:892–910.
Trotta M, Debernardi F, Caputo O. Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int J Pharm Elsevier. 2003;257:153–60.
Pizzol C, Filippin-Monteiro F, Restrepo J, Pittella F, Silva A, Alves de Souza P, et al. Influence of surfactant and lipid type on the physicochemical properties and biocompatibility of solid lipid nanoparticles. Int J Environ Res Public Health. 2014;11:8581–96.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–37.
Essa E. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J Pharm. 2010;4:227–33.
Svilenov H, Tzachev C. Solid lipid nanoparticles - a promising drug delivery system. Nanomedicine. One Central Press Manchester. 2014. p. 187–237.
Verheyen S, Augustijns P, Kinget R, Van den Mooter G. Determination of partial solubility parameters of five benzodiazepines in individual solvents. Int J Pharm. 2001;228:199–207.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm Elsevier. 2001;226:147–61.
Bordes C, Fréville V, Ruffin E, Marote P, Gauvrit JY, Briançon S, et al. Determination of poly(ɛ-caprolactone) solubility parameters: application to solvent substitution in a microencapsulation process. Int J Pharm. 2010;383:236–43.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLNTM) dispersions. Int J Pharm Elsevier. 1998;168:221–9.
Schwarz C. Solid lipid nanoparticles (SLN) for controlled drug delivery II. drug incorporation and physicochemical characterization. J Microencapsul. Taylor & Francis. 1999;16:205–13.
Taylor LS, York P. Characterization of the phase transitions of trehalose dihydrate on heating and subsequent dehydration. J Pharm Sci. 1998;87:347–55.
Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics. 2021;13.
Singh A, Neupane YR, Mangla B, Kohli K. Nanostructured lipid carriers for oral bioavailability enhancement of exemestane: formulation design, in vitro, ex vivo, and in vivo studies. J Pharm Sci Elsevier Ltd. 2019;108:3382–95.
Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63:288–94.
Sha X, Yan G, Wu Y, Li J, Fang X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur J Pharm Sci. 2005;24:477–86.
Acknowledgements
The authors express their sincere gratitude towards the All India Council for Technical Education (AICTE-NAFETIC) to offer research facilities. Authors are also thankful to Gattefosse, India, Mohini Organics Pvt. Ltd. India, Abitec Corporation, India, and BASF, India, for providing gift samples of various excipients.
Funding
This research is financially supported by the University Grants Commission of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shevalkar, G., Pawar, M. & Vavia, P. Nanostructured Lipid Carriers (NLCs) of Lumefantrine with Enhanced Permeation. J Pharm Innov 17, 1221–1234 (2022). https://doi.org/10.1007/s12247-021-09590-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09590-1