Abstract
Purpose
Powder properties, such as density and adhesion, can cause large variability in the flow rate of ingredients fed from feeders, which can propagate through the system. Knowing an ideal range of operation and correlating powder properties to process performance can result in faster optimization of performance, which saves time and money.
Method
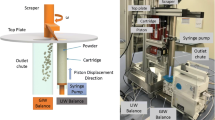
A K-Tron KT-20 pharmaceutical loss-in-weight feeder was evaluated with multiple screw types, feed rates, and materials. The material was dispensed onto a catch scale, recording mass versus time, and analyzed for relative standard deviation and deviation from the setpoint. The materials used in this work were characterized by particle size, packing, and flow properties.
Results
Linear relationships between the feeder’s throughput capacity and material bulk density were identified for four different types of screws. Analyzing the feeder data resulted in a region where the RSD was at a minimum across a range of screw speeds. This was identified between 20–90% screw speed for coarse concave, fine concave, and coarse auger screws and 40–90% screw speed for fine auger screws.
Conclusion
An optimal range of operation for K-Tron KT20 feeders was identified, as well as a linear correlation between material conditioned bulk density and maximum throughput for a given material and screw type. Using both the predictable maximum throughput and the optimal screw range, an optimal design space has been identified that will save development time and money.










Similar content being viewed by others
References
Wilson DH, Bullivant K W, Loss-in-weight gravimetric feeder. Google Patents: 1986.
Hopkins M. Loss in weight feeder systems. Measurement and Control. 2006;39(8):237–40.
Weinekötter R, Reh L. Continuous mixing of fine particles. Part Part Syst Charact. 1995;12(1):46–53.
Engisch W, Ierapetritou M, Muzzio F. Hopper refill of loss-in-weight feeding equipment. Salt Lake City, UT, USA: Proceedings of the 2010 AIChE Annual Meeting; 2010.
Plumb K. Continuous processing in the pharmaceutical industry: changing the mindset. Chem Eng Res Des. 2005;83(6):730–8.
Schaber SD, Gerogiorgis DI, Ramachandran R, Evans JM, Barton PI, Trout BL. Economic analysis of integrated continuous and batch pharmaceutical manufacturing: a case study. Ind Eng Chem Res. 2011;50(17):10083–92.
Leuenberger H. New trends in the production of pharmaceutical granules: batch versus continuous processing. Eur J Pharm Biopharm. 2001;52(3):289–96.
Roberge DM, Zimmermann B, Rainone F, Gottsponer M, Eyholzer M, Kockmann N. Microreactor technology and continuous processes in the fine chemical and pharmaceutical industry: is the revolution underway? Org Process Res Dev. 2008;12(5):905–10.
Editors PT, FDA approves tablet production on Janssen continuous manufacturing line. PharmTech.com 2016. Accessed 8 April 2016.
Food Administration D. Guidance for industry PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Rockville, MD: DHHS; 2004.
Lawrence XY. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91.
Rathore AS. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009;27(9):546–53.
Urquhart L. Regulatory watch: FDA new drug approvals in Q1 2018. Nat Publ Group. 2018;17(5):309.
Lee S. Current FDA perspective for continuous manufacturing. Cambridge, Vereinigte Staaten von Amerika: 2nd International Symposium on Continuous Manufacturing of Pharmaceuticals; 2016.
Walker N, EMA approves continuous manufacture of prezista. Pharma’s Almanac 2017, PAO-M07-17-NI-002.
2016 ISPE Continous manufacturing conference summary. 2017.
Johnson NR, System for precisely controlling discharge rates of loss-in-weight feeder systems. Google Patents 1992.
Aalto P; Björklund J-P, Loss-in-weight feeder control. Google Patents 2002.
Kehlenbeck V, Sommer K. Possibilities to improve the short-term dosing constancy of volumetric feeders. Powder Technol. 2003;138(1):51–6.
Tardos GI, Lu Q. Precision dosing of powders by vibratory and screw feeders: an experimental study. Adv Powder Technol. 1996;7(1):51–8.
Engisch WE, Muzzio FJ. Method for characterization of loss-in-weight feeder equipment. Powder Technol. 2012;228:395–403.
Engisch WE, Muzzio FJ. Feedrate deviations caused by hopper refill of loss-in-weight feeders. Powder Technol. 2015;283:389–400.
Cordts E, Steckel H. Capabilities and limitations of using powder rheology and permeability to predict dry powder inhaler performance. Eur J Pharm Biopharm. 2012;82(2):417–23.
Fu X, Huck D, Makein L, Armstrong B, Willen U, Freeman T. Effect of particle shape and size on flow properties of lactose powders. Particuology. 2012;10(2):203–8.
Bharadwaj R, Ketterhagen WR, Hancock BC. Discrete element simulation study of a Freeman powder rheometer. Chem Eng Sci. 2010;65(21):5747–56.
Huang Z, Scicolone JV, Gurumuthy L, Davé RN. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: a continuous dry coating device. Chem Eng Sci. 2015;125:209–24.
Huang Z, Scicolone JV, Han X, Davé RN. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int J Pharm. 2015;478(2):447–55.
Scicolone JV, Metzger M, Koynov S, Anderson K, Takhistov P, Glasser BJ, et al. Effect of liquid addition on the bulk and flow properties of fine and coarse glass beads. AICHE J. 2016;62(3):648–58.
Wang Y, Koynov S, Glasser BJ, Muzzio FJ. A method to analyze shear cell data of powders measured under different initial consolidation stresses. Powder Technol. 2016;294:105–12.
Freeman R. Measuring the flow properties of consolidated, conditioned and aerated powders—a comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007;174(1):25–33.
Carson JW, Wilms H. Development of an international standard for shear testing. Powder Technol. 2006;167(1):1–9.
Peschl I. New rotational shear-testing technique. J Powder Bulk Solids Technol. 1977;1(3):55.
Schwedes J, Schulze D. Measurement of flow properties of bulk solids. Powder Technol. 1990;61(1):59–68.
Schmitt R, Feise H. Influence of tester geometry, speed and procedure on the results from a ring shear tester. Part Part Syst Charact: Measurement and Description of Particle Properties and Behavior in Powders and Other Disperse Systems. 2004;21(5):403–10.
Acknowledgements
The authors would like to thank Nikita Soni, Glinka Pereira, Kien Chau, Lan Le, and Nikhita Shetty for the assistance in this work.
Funding
The research was financially supported by the Janssen Ortho LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Scicolone, J.V., Sanchez, E. et al. Identifying a Loss-in-Weight Feeder Design Space Based on Performance and Material Properties. J Pharm Innov 15, 482–495 (2020). https://doi.org/10.1007/s12247-019-09394-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09394-4