Abstract
Purpose
Asenapine maleate (ASP) is an antipsychotic agent used in the treatment of schizophrenia and bipolar disorder. It has extremely low oral bioavailability of < 2%, necessitating the utilization of alternate route of administration. The objective of this work is to study and optimize the sonophoretic transdermal delivery and skin retention of ASP statistically, in Sprague-Dawley rat skin, using response surface methodology in Design of Experiments (DoE).
Method
I-optimal design was employed, using the ultrasound (US) parameters viz., duration of US application, amplitude of US, and mode of US application (simultaneous application or pretreatment) as the input variables. Steady-state flux (Jss) of ASP and amount of drug retained in skin after 24 h was taken as the output responses.
Results
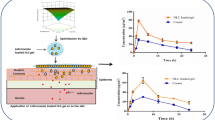
The model and dependent variables were found to be significant and representative of the data and response surface. While passive diffusion yielded Jss of 2.575 μg/cm2/h, the same values with US application ranged from 8.18 to 127.68 μg/cm2/h. Passive diffusion of drug showed 46.22 ± 5.2 μg/cm2 of ASP retained in 24 h, while US application resulted in 99.07 to 1495.6 μg/cm2 of drug retained in skin in 24 h. Based on the findings from optimization studies, 30 min of US application, with amplitude of 27–28, and simultaneous application mode was found to achieve the optimal transdermal drug flux, with slightly lower retention values in skin.
Conclusion
The study found that the sonophoretic transdermal permeation and retention of ASP in vitro could be optimized using response surface methodology.





Similar content being viewed by others
References
Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10:893–903.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_ChemR.pdfWO2010127674A1. Accessed on 27-09-2018.
Van Der Sterren JEM, Van Den Heuvel DJM. Intranasal administration of asenapine and pharmaceutical compositions therefore. United State Patent US 2008/0306133 A1. 2008
Solomon WD. Transdermal compositions of asenapine for the treatment of psychiatric disorders. WO2010127674A1. 2009.
Shreya AB, Managuli RS, Menon J, Kondapalli L, Hegde AR, Avadhani K, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: in vitro and in vivo performance evaluations. J Liposome Res. 2016;26:221–32.
Singh SK, Dadhania P, Vuddanda PR, Jain A, Velaga S, Singh S. Intranasal delivery of asenapine loaded nanostructured lipid carriers: formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016;6:2032–45.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Shetty PK, et al. Transdermal delivery of lercanidipine hydrochloride: effect of chemical enhancers and ultrasound. Curr Drug Deliv. 2013;10:427–34.
Manikkath J, Hegde AR, Kalthur G, Parekh HS, Mutalik S. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int J Pharm. 2017;521:110–9.
Dragicevic N, Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev. 2018;127:58–84.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–18.
Baji S, Hegde AR, Kulkarni M, Raut SY, Manikkath J, Reddy MS, et al. Skin permeation of gemcitabine hydrochloride by passive diffusion, iontophoresis and sonophoresis: in vitro and in vivo evaluations. J Drug Delivery Sci Technol. 2018;47:49–54.
Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152:330–48.
Mitragotri S, Kost J. Low-frequency sonophoresis: a noninvasive method of drug delivery and diagnostics. Biotechnol Prog. 2002;16:488–92.
Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243:1–15.
Herwadkar A, Sachdeva V, Taylor LF, Silver H, Banga AK. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int J Pharm. 2012;423:289–96.
Kumar S, Malick AW, Meltzer NM, Mouskountakis JD, Behl CR. Studies of in vitro skin permeation and retention of a leukotriene antagonist from topical vehicles with a hairless Guinea pig model. J Pharm Sci. 1992;81:631–4.
Singh B, Kapil R, Nandi M, Ahuja N. Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects. Expert Opin Drug Deliv. 2011;8:1341–60.
Politis NS, Colombo P, Colombo G, Rekkas MD. Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm. 2017;43:889–901.
Huang B, Dong WJ, Yang GY, Wang W, Ji CH, Zhou FN. Dendrimer-coupled sonophoresis-mediated transdermal drug-delivery system for diclofenac. Drug Des Devel Ther. 2015;23(9):3867–76.
Managuli RS, Kumar L, Chonkar AD, Shirodkar RK, Lewis S, Koteshwara KB, et al. Development and validation of a stability-indicating RP-HPLC method by a statistical optimization process for the quantification of asenapine maleate in lipidic nanoformulations. J Chromatogr Sci. 2016;54:1290–300.
Manikkath J, Manikkath A, Shavi GV, Bhat K, Mutalik S. Low frequency ultrasound and PAMAM dendrimer facilitated transdermal delivery of ketoprofen. J Drug Delivery Sci Technol. 2017;41:334–43.
Mutalik S, Nayak UY, Kalra R, Kumar A, Kulkarni RV, Parekh HS. Sonophoresis-mediated permeation and retention of peptide dendrimers across human epidermis. Skin Res Technol. 2012;18:101–7.
Mutalik S, Parekh HS, Davies NM, Nayanabhirama U. A combined approach of chemical enhancers and sonophoresis for the transdermal delivery of tizanidine hydrochloride. Drug Deliv. 2009;16:82–91.
Mitragotri S, Farrell J, Tang H, Terahara T, Kost J, Langer R. Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J Control Release. 2000a;63:41–52.
Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–60.
Manikkath J, Sumathy TK, Manikkath A, Mutalik S. Delving deeper into dermal and transdermal drug delivery: factors and mechanisms associated with nanocarrier-mediated strategies. Curr Pharm Des. 2018;24:3210–22.
Tang H, Mitragotri S, Blankschtein D, Langer R. Theoretical description of transdermal transport of hydrophilic permeants: application to low-frequency sonophoresis. J Pharm Sci. 2001;90:545–68.
Tachibana K, Tachibana S. Transdermal delivery of insulin by ultrasonic vibration. J Pharm Pharmacol. 1991;43:270–1.
Tezel A, Sens A, Tuchscherer J, Mitragotri S. Synergistic effect of low-frequency ultrasound and surfactants on skin permeability. J Pharm Sci. 2002;91:91–100.
Kim D-D, Chien YW. Transdermal delivery of dideoxynucleoside-type anti-HIV drugs. 2. The effect of vehicle and enhancer on skin permeation. J Pharm Sci. 1996;85:214–9.
Mutalik S, Udupa N. Effect of some penetration enhancers on the permeation of glibenclamide and glipizide through mouse skin. Pharmazie. 2003;58:891–4.
https://www.ema.europa.eu/documents/assessment-report/sycrest-epar-public-assessment-report_en.pdf. Accessed on 27-09-2018.
Acknowledgements
The authors are thankful to Orbicular Pharmaceutical Technologies Pvt. Ltd., Hyderabad, India for the gift sample of asenapine maleate, and to Manipal Academy of Higher Education, Manipal, India, for providing the facilities required to conduct the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manikkath, J., Shenoy, G.G., Pandey, S. et al. Response Surface Methodology for Optimization of Ultrasound-Assisted Transdermal Delivery and Skin Retention of Asenapine Maleate. J Pharm Innov 14, 391–399 (2019). https://doi.org/10.1007/s12247-019-09386-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09386-4