Abstract
Purpose
With the applications of more advanced manufacturing technologies being applied to the pharmaceutical industry, continuous processes are at the forefront of innovation. One area that is highly desired to be systematically investigated is material traceability in continuous manufacturing systems. By following federal guidelines already in place, the goal was to address the issue of material traceability in a continuous direct compression tablet manufacturing process.
Methods
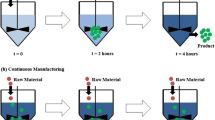
The residence time distribution (RTD) method has been used for material traceability in continuous pharmaceutical tablet manufacturing process. Utilizing the minimum and maximum residence times for the continuous line pre-production, raw material batch changes that occur during feeder refill can be traced at the outlet of the process. MATLAB programing was used to develop software prototype to trace material components.
Results
Developed framework for implementation of material traceability into continuous manufacturing pilot-plant. To demonstrate the application of this framework, a software prototype was developed, which allows the operator to input residence time attributes for each component in the formulation. Using the minimum and maximum residence time values for that component, the lot number is incremented when the change in material batch is predicted to be present in the tablets at the outlet. The tablet lot number is recorded by the control system in real-time.
Conclusions
Developed framework and corresponding software allows the material traceability to be fully accounted for during a continuous drug product manufacturing process. A proof of concept was created to demonstrate feasibility of such a system, which has a wide range of applications.















Similar content being viewed by others
References
Subcommitte for Advanced Manufacgturing of the National Science and Technology Council. Advanced manufacturing: a snapshot of priority technology areas across the federal government. United States Gov. 2016;1–63. Available from: https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Blog/NSTCSAMtechnologyareassnapshot.pdf. Accessed 22 Dec 2017.
Singh R, Muzzio F, Ierapetritou M, Ramachandran R. A combined feed-forward/feed-Back control system for a QbD-based continuous tablet manufacturing process. Processes. 2015;3:339–56.
Engisch WE, Muzzio FJ. Method for characterization of loss-in-weight feeder equipment. Powder Technol. Elsevier B.V. 2012;228:395–403.
FDA. CFR—Code of Federal Regulations Title 21. The information on this page is current as of April 1 2015. 2015;3:25–6. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=314.80. Accessed 23 May 2018.
Lundqvist S-O, Kubulnieks E. Improved production and product information for chemical pulp mill operators. IFAC Proc. 1995;28:19–24.
Moe T. Perspectives on traceability in food manufacture. Trends Food Sci Technol. 1998;9:211–4.
Mousavi A, Sarhadi M, Lenk A, Fawcett S. Tracking and traceability in the meat processing industry: a solution. Br Food J Emerald. 2002;104:7–19.
Jansen-Vullers MH, van Dorp CA, Beulens AJM. Managing traceability information in manufacture. Int J Inf Manag. 2003;23:395–413.
Folinas D, Manikas I, Manos B. Traceability data management for food chains. Br Food J Emerald. 2006;108:622–33.
Kvarnström B, Oghazi P. Methods for traceability in continuous processes—experience from an iron ore refinement process. Miner Eng. 2008;21:720–30.
Engisch W, Muzzio F. Using residence time distributions (RTDs) to address the traceability of raw materials in continuous pharmaceutical manufacturing. J Pharm Innov. 2016;11:64–81.
Williams JC, Rahman MA. Prediction of the performance of continuous mixers for particulate solids using residence time distributions: part II. Experimantal. Powder Technol. 1972;5:307–16.
Singh R, Ierapetritou M, Ramachandran R. System-wide hybrid MPC-PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur J Pharm Biopharm Elsevier B.V. 2013;85:1164–82.
Vanarase AU, Muzzio FJ. Effect of operating conditions and design parameters in a continuous powder mixer. Powder TechnolElsevier B.V. 2011;208:26–36.
Gao Y, Vanarase A, Muzzio F, Ierapetritou M. Characterizing continuous powder mixing using residence time distribution. Chem Eng Sci Elsevier. 2011;66:417–25.
Vanarase AU, Alcalà M, Jerez Rozo JI, Muzzio FJ, Romañach RJ. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65:5728–33.
Wang Z, Escotet-Espinoza MS, Ierapetritou M. Process analysis and optimization of continuous pharmaceutical manufacturing using flowsheet models. Comput Chem EngElsevier Ltd. 2017;107:77–91.
Boukouvala F, Niotis V, Ramachandran R, Muzzio FJ, Ierapetritou MG. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process. Comput Chem Eng Elsevier Ltd. 2012;42:30–47.
Want R. An introduction to RFID technology. IEEE Pervasive Comput. 2006;5:25–33.
Funding
This work is supported by the Rutgers Research Council, through grant 202342 RC-17-Singh R, and National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, through Grant NSF-ECC 0540855.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billups, M., Singh, R. Systematic Framework for Implementation of Material Traceability into Continuous Pharmaceutical Tablet Manufacturing Process. J Pharm Innov 15, 51–65 (2020). https://doi.org/10.1007/s12247-018-9362-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9362-9