Abstract
Purpose
During continuous manufacturing, there may be some out of specification tablets that need to be diverted in real time, in order to ensure the quality of the final product. Specifically, the content uniformity of each tablet must be guaranteed before it can be released to market. However, currently, no methods or tools are available that can assure the content uniformity and divert the non-confirming products in real time. The aim of this work is to develop and evaluate a strategy to divert the non-confirming tablets in real time and thereby assure drug concentration of final tablets.
Methods
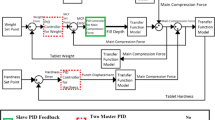
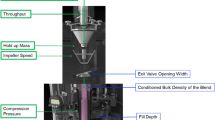
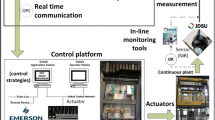
This work has been conducted in silico using a combination of MATLAB and Simulink. A methodology to implement a residence time distribution (RTD)-based control system for drug concentration-based tablet diversion which uses the convolution integral was developed and implemented in MATLAB. Comparisons between the performance of “fixed window” and “RTD-based” approaches for diversion have also been presented and used to assess optimal usability.
Results
In this work, two novel strategies namely, “fixed window approach” and “RTD-based approach” have been developed and evaluated for real-time diversion of non-confirming tablets. The RTD-based control system was designed, developed, and implemented in silico. A framework for its implementation in a real-time system has also been elaborated on. This methodology was compared to an alternative fixed window approach. The proposed control system is analyzed for various manufacturing scenarios, systems, and disturbances.
Conclusions
A comparison of the two proposed strategies suggests that the “RTD-based control system” is more efficient in every simulated scenario. The relative performance is best when the disturbances in the system are characterized by short pulse-like changes.












Similar content being viewed by others
References
Lee SL, O’Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov. 2015;10(3):191–9.
Plumb K. Continuous processing in the pharmaceutical industry: changing the mind set. Chem Eng Res Des. 2005;83(6):730–8.
FDA. Guidance for industry PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance.
Bhaskar A, Barros FN, Singh R. Development and implementation of an advanced model predictive control system into continuous pharmaceutical tablet compaction process. Int J Pharm. 2017;534(1–2):159–78.
Nunes de Barros F, Bhaskar A, Singh R. A validated model for design and evaluation of control architectures for a continuous tablet compaction process. vol. 5, Processes. 2017
Su Q, Moreno M, Giridhar A, Reklaitis GV, Nagy ZK. A systematic framework for process control design and risk analysis in continuous pharmaceutical solid-dosage manufacturing. J Pharm Innov. 2017;12(4):327–46.
Singh R, Muzzio JF, Ierapetritou M, Ramachandran R. A Combined feed-forward/feed-back control system for a QbD-based continuous tablet manufacturing process, vol. 3, Processes. 2015.
Singh R, Ierapetritou M, Ramachandran R. System-wide hybrid MPC–PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur J Pharm Biopharm. 2013;85(3, Part B):1164–82.
Ramachandran R, Arjunan J, Chaudhury A, Ierapetritou MG. Model-based control-loop performance of a continuous direct compaction process. J Pharm Innov. 2011;6(4):249–63.
Singh R, Ierapetritou M, Ramachandran R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. Int J Pharm. 2012;438(1):307–26.
Hsu S-H, Reklaitis GV, Venkatasubramanian V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: process dynamics and control framework. J Pharm Innov. 2010;5(1):14–23.
Mesbah A, Paulson JA, Lakerveld R, Braatz RD. Model predictive control of an integrated continuous pharmaceutical manufacturing pilot plant. Org Process Res Dev. 2017;21(6):844–54.
Lakerveld R, Benyahia B, Heider PL, Zhang H, Wolfe A, Testa CJ, et al. The application of an automated control strategy for an integrated continuous pharmaceutical pilot plant. Org Process Res Dev. 2015;19(9):1088–100.
Singh R, Muzzio FJ, Ierapetritou M, Ramachandran R. Integrated control and data management system for continuous pharmaceutical anufacturing process. Oral presentation at AIChE annual meeting, Minneapolis, MN, 29 October - 3 November. 2017.
Gao Y, Vanarase A, Muzzio F, Ierapetritou M. Characterizing continuous powder mixing using residence time distribution. Chem Eng Sci. 2011;66(3):417–25.
Gao Y, Muzzio FJ, Ierapetritou MG. A review of the residence time distribution (RTD) applications in solid unit operations. Powder Technol. 2012;228:416–23.
Abouzeid A-ZMA, Mika TS, Sastry KV, Fuerstenau DW. The influence of operating variables on the residence time distribution for material transport in a continuous rotary drum. Powder Technol. 1974;10(6):273–88.
Mateo-Ortiz D, Méndez R. Relationship between residence time distribution and forces applied by paddles on powder attrition during the die filling process. Powder Technol. 2015;278:111–7.
Engisch W, Muzzio F. Using residence time distributions (RTDs) to address the traceability of raw materials in continuous pharmaceutical manufacturing. J Pharm Innov. 2016 Nov 14;11:64–81.
Fogler H. Elements of chemical reaction engineering. 2006.
Singh R, Sahay A, Karry KM, Muzzio F, Ierapetritou M, Ramachandran R. Implementation of an advanced hybrid MPC–PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. Int J Pharm. 2014;473(1):38–54.
Funding
This work is supported by the Rutgers Research Council, through grant 202342 RC-17-Singh R; the US Food and Drug Administration (FDA), through grant 11695471; and the National Science Foundation Engineering Research Center on Structured Organic Particulate Systems, through grant NSF-ECC 0540855.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Nomenclature
Abbreviations | Variable |
|---|---|
API | Active pharmaceutical ingredient |
CSTR | Continuous stirred tank reactor |
CQA | Critical quality attributes |
NIR | Near infrared |
PFR | Plugged flow reactor |
PAT | Process analytical technology |
RTRT | Real-time release testing |
RTD | Residence time distribution |
Symbol | Variable | Units |
|---|---|---|
C | Concentration | g/m3 |
C(t) | Concentration at time t | (%) |
E(t) | Residence time distribution function | s−1 |
F(t) | Cumulative distribution function | (−) |
n | Number of tanks | (−) |
t | Time | s |
ε | Manufacturing efficiency | % |
σ | Variance | s |
τ | Mean residence time | s |
Subscript | Variable |
|---|---|
a | Accept |
di | Initial delay |
df | Final delay |
exp | Experimental |
f | Concentration after step change |
in | Input stream |
o | Off-specification |
out | Output stream |
s | Specification |
r | Reject |
Rights and permissions
About this article
Cite this article
Bhaskar, A., Singh, R. Residence Time Distribution (RTD)-Based Control System for Continuous Pharmaceutical Manufacturing Process. J Pharm Innov 14, 316–331 (2019). https://doi.org/10.1007/s12247-018-9356-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9356-7