Abstract
Purpose
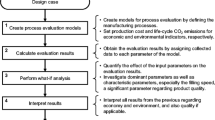
There is a continuous search for efficient optimization methods in the production of biopharmaceuticals, with a special focus on reducing production time and costs. Pharmaceutical production is currently dominated by batch production processes that have a relatively long downtime for cleaning and reconfiguration of the plant equipment when the product or its format size is changed. The geometry and design of the equipment can strongly affect process performance. This work presents an approach for generating and assessing design alternatives for process equipment used in the sterile filling of biopharmaceutical drug products.
Methods
A case study is presented based on background information provided by Hoffmann-La Roche for a surge tank used in the filling of liquid vials. Three criteria—namely equipment cleanability, production efficiency, and flexibility—are used to evaluate the proposed shape alternatives for the surge tank. Computational fluid dynamics (CFD) was applied to investigate the flow behavior of cleaning water inside the tank.
Results
A trade-off in the results was observed between the different evaluation criteria for the investigated basic shapes. However, it was found that a different part of the tank controls the results obtained for each criterion. An alternative tank design is presented that combines the optimal features to achieve the best possible performance for all assessment criteria.
Conclusion
The transfer and application of the established CFD technique to the field of sterile manufacturing of biopharmaceuticals were demonstrated and facilitated by the introduction of appropriate model simplifications. Valuable insights on equipment cleanability and on the measures required to achieve cleanliness were obtained, which proves the importance of the application of such techniques. Redesigning equipment and applying geometrical changes were shown to be effective measures for process performance optimization.











Similar content being viewed by others
Abbreviations
- alt.:
-
Alternative
- CAD:
-
Computer-aided design
- CFD:
-
Computational fluid dynamics
- CIP:
-
Cleaning-in-place
- FVM:
-
Finite volume method
- OpenFOAM :
-
Open source field operation and manipulation
- PSE:
-
Process system engineering
- RANS:
-
Reynolds-averaged Navier–Stokes equation
- RAS:
-
Reynolds-averaged simulation
- SIP:
-
Sterilization-in-place
- SST:
-
Shear stress transport
- VOF:
-
Volume of fluid
- WFI:
-
Water-for-injection
- ε :
-
Turbulent dissipation (m2·s−3)
- k :
-
Turbulent kinetic energy (m2·s−2)
- Γ :
-
Percentage of wetting (−)
- ω :
-
Specific turbulence dissipation rate (s−1)
- P loss :
-
Product loss (m3/batch)
- t :
-
Time (s)
- u :
-
Velocity vector in (x, y, z)-direction (m·s−1)
- u’ :
-
Small oscillations in (x, y, z)-direction (m·s−1)
- U :
-
Time-averaged velocity vector in (x, y, z)-direction (m·s−1)
- V flex :
-
Volume representing the production flexibility (m3)
- V max :
-
Volume up to the maximum liquid level (m3)
- V min :
-
Volume up to the minimum liquid level (m3)
References
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14.
Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov Nat Publ Group. 2012;11:191–200.
Graham LJ, Taillon R, Mullin J, Wigle T. Pharmaceutical process/equipment design methodology case study: cyclone design to optimize spray-dried-particle collection efficiency. Comput Chem Eng Elsevier Ltd. 2010;34:1041–8.
Shirahata H, Hirao M, Sugiyama H. Multiobjective decision-support tools for the choice between single-use and multi-use technologies in sterile filling of biopharmaceuticals. Comput Chem Eng 2018
Chisti Y, Moo-Young M. Clean-in-place systems for industrial bioreactors: design, validation and operation. J Ind Microbiol Springer-Verlag. 1994;13:201–7.
Behr A, Brehme VA, Ewers CLJ, Grön H, Kimmel T, Küppers S, et al. New developments in chemical engineering for the production of drug substances. Eng Life Sci. 2004;4:15–24.
Meyer BK, Coless L. Compounding and filling: drug substance to drug product. Ther Protein Drug Prod. Woodhead Publishing; 2012. p. 83–95.
Tamime AY. Cleaning-in-place: dairy, food and beverage operations. Blackwell Publishing; 2008.
Norton T, Sun D-W, Grant J, Fallon R, Dodd V. Applications of computational fluid dynamics (CFD) in the modelling and design of ventilation systems in the agricultural industry: a review. Bioresour Technol. 2007;98:2386–414.
Karama AB, Onyejekwe OO, Brouckaert CJ, Buckley CA. The use of computational fluid dynamics (CFD). Technique for evaluating the efficiency of an activated sludge reactor. Water Sci Technol. 1999;39:329–32.
Franke J, Hirsch C, Jensen AG, Krus HW, Schatzmann M, Westbury PS, et al. Recommendations on the use of CFD in wind engineering. Cost Action. 2004;C14:1–11.
Löffelholz C. CFD als Instrument zur bioverfahrenstechnischen Charakterisierung von Single-use Bioreaktoren und zum Scale-up für Prozesse zur Etablierung und Produktion von Biotherapeutika. PhD Thesis, Brandenburg Unversity of Technology. 2013.
Norton T, Sun D-W. Computational fluid dynamics (CFD)—an effective and efficient design and analysis tool for the food industry: a review. Trends Food Sci Technol. 2006;17:600–20.
Friis A, Jensen BBB. Prediction of hygiene in food processing equipment using flow modelling. Trans IChemE Elsevier. 2002;80:281–5.
Wassgren C, Curtis JS. The application of computational modeling to pharmaceutical materials science. MRS Bull Cambridge Univ Press. 2006;31:900–4.
Tong ZB, Zheng B, Yang RY, Yu AB, Chan HK. CFD-DEM investigation of the dispersion mechanisms in commercial dry powder inhalers. Powder Technol. 2013;240:19–24.
Casola G, Siegmund C, Mattern M, Sugiyama H. Uncertainty-conscious methodology for process performance assessment in biopharmaceutical drug product manufacturing. AIChE J. 2018;64:1272–84.
Yeckel A, Middleman S. Removal of a viscous film from a rigid plane surface by an impinging liquid jet. Chem Eng Commun. 1987;50:165–75.
Knupp PM. Algebraic mesh quality metrics. SIAM J Sci Comput. 2001;23:193–218.
Schlipf M, Tismer A, Riedelbauch S. On the application of hybrid meshes in hydraulic machinery CFD simulations. IOP Conf Ser Earth Environ Sci. 2016;49:1–10.
Schöberl J. An advancing front 2D/3D-mesh generator based on abstract rules. Comput Vis Sci. 1997;1:41–52.
Weller HG, Tabor G, Jasak H, Fureby C. A tensorial approach to computational continuum mechanics using object-oriented techniques. J Comput Phys. 1998;12:620–31.
Greenshields C. OpenFOAM the OpenFOAM Foundation user guide. 2011.
Hirt C, Nichols B. Volume of fluid (VOF) method for the dynamics of free boundaries. J Comput Phys. 1981;39:201–25.
Sigloch H. Technische Fluiddynamik. Springer; 2014.
Kajishima T, Taira K. Reynolds-averaged Navier–Stokes equations. Comput Fluid Dyn. Springer; 2017. p. 237–68.
Wilcox DC. Formulation of the k-omega turbulence model revisited. AIAA J. 2008;46:2823–38.
Frei W. Which turbulence model should I choose for my CFD application?. 2017.
Menter FR. Zonal two equation k-w, turbulence models for aerodynamic flows. AIAA Pap 1993;2906.
Menter FR. Two-equation eddy-viscosity turbulence models for engineering applications. AIAA J. 1994;32:1598–605.
https://www.openfoam.com/documentation/cpp-guide/html/guide-schemes-wall-distance-meshwave.html. Accessed 25 June 2018.
https://www.cfd-online.com/Wiki/Turbulence_free-stream_boundary_conditions. Accessed 25 June 2018.
Hasting APM. Designing for cleanability. Cleaning-in-place dairy, food beverage oper. John Wiley & Sons; 2008.
Morison K, Thorpe R. Liquid distribution from cleaning-in-place sprayballs. Food Bioprod Process. 2002;80:2–7.
Acknowledgments
The authors would like to thank the many collaborators from F. Hoffmann–La Roche Ltd for providing necessary information and resources for this study. The authors also acknowledge financial support from a Grant-in-Aid for Young Scientists (A) No. 17H04964 from the Japan Society for the Promotion of Science, and a Research Grant 2017 from the Nagai Foundation Tokyo. The Top Global University Project of the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and Japan Student Services Organization (JASSO) are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Teaser
The work presents an approach to redesign equipment in the filling step of biopharmaceutical drug product manufacturing. It investigates the effect of the shape of the equipment on process performance and introduces an application of computational fluid dynamics to simulate the cleaning-in-place process.
Rights and permissions
About this article
Cite this article
Zeberli, A., Casola, G., Badr, S. et al. Approach for Multicriteria Equipment Redesign in Sterile Manufacturing of Biopharmaceuticals. J Pharm Innov 15, 15–25 (2020). https://doi.org/10.1007/s12247-018-9355-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9355-8