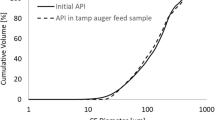
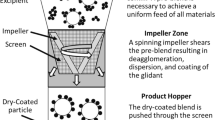
Abstract
Purpose
A dry coating technology has been evaluated as a dry agglomeration technique for a typical pharmaceutical formulation. Its efficiency was compared with that of the more commonly used roller compaction dry granulation.
Methods
A commercially available system was selected as a representative technology. The selection was based on batch size, processing time, unit size, vendor support, preventive maintenance requirements, development stage, and maturity for pharmaceutical industry requirements. The comparison targets the behavior of the resulting materials in terms of flowability, compressibility, mixing efficiency, and prevention of segregation. Particle size distribution, bulk density, scanning electron microscopy, FT4 powder rheometer, and hardness were used for the characterization of the powders. A design of experiment was used to evaluate the impact of the dry coating equipment operating parameters (rotor speed and processing time) as well as the impact of formulation (grade of microcrystalline cellulose and ratio between dibasic calcium phosphate and lactose).
Results
The tested dry coating technology shows (1) an improved compressibility, (2) that the powders are homogenous, and (3) that they do not have a tendency to segregate in downstream process steps and during handling and storage. The impact of the rotor speed is proven statistically not significant, but it seems that a lower rotor speed might lead to less attrition and better flow properties. Processing time does not seem to have an impact for the range of processing times evaluated. Compared to roller compaction, the main advantages of the tested technology are the shape and surface enhancement and the absence of work hardening.
Conclusions
This work has shown that the blends produced by the targeted dry coating equipment has equal or better results than the powders processed by roller compaction for all the critical quality attributes that were evaluated.




























Similar content being viewed by others
References
Osborne JD, Althaus T, Forny L, Niederreiter G, Palzer S, Hounslow MJ, et al. Investigating the influence of moisture content and pressure on the bonding mechanisms during roller compaction of an amorphous material. Chem Eng Sci. 2013;86:61–9, 2.
Teng Y, Qiu Z, Wen H. Systematical approach of formulation and process development using roller compaction. Eur J Pham Biopharm. 2009;73:219–29, 10.
Miller RW. In: Parikh DM, editor. Handbook of pharmaceutical granulation. Didcot: Taylor and Francis; 2005. p. 159–90.
Gessel S, Druinen H, Bogaerts I. Roller compaction of anhydrous lactose and blends of anhydrous lactose with MCC, 2009.
Kleinebudde P. Roll compaction/dry granulation: pharmaceutical applications. Eur J Pham Biopharm. 2004;58:317–26.
Perez-Gandarillas L, Mazor A, Souriou D, Lecoq O, Michrafy A. Compaction behaviour of dry granulated binary mixtures. Powder Technol. 2015;285:62–7, 11.
Hosokawa Micron, Nano Particle Technology and Particle Design, extracted from https://www.hosokawa.co.uk/wpcontent/uploads/2014/03/Nano_Particle_Technology__Particle_Design.pdf, 2004.
Nara Machinery co. ltd. Nara Hybridization System Powder surface modification technology, extracted from http://www.aaamachine.com/products/other/pdf/Hybridization_NHS_NaraMachinery.pdf, 2011
Pfeffer R, Dave RN, Wei D, Ramlakhan M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001;117:40–67.
Saharan VA, Kukkar V, Kataria M, Kharb V, Choudhury PK. Ordered mixing: mechanism, process and application in pharmaceutical formulations. Asian J Pharm Sci. 2008;3:240–59.
Hersey JA. Ordered mixing: a new concept in powder mixing practice. Powder Technol. 1975;11:41–4.
Parikh DM. In: Parikh DM, editor. Handbook of pharmaceutical granulation. Didcot: Taylor and Francis; 2005. p. 1–6.
Pietsch W. Agglomeration processes: phenomena, technologies, equipment, J. Wiley and Sons, Éds., Wiley-VCH, Hoboken, 2002.
Alonso M, Alguacil FJ. Stochastic modeling of particle coating. AICHE J. 2001;47:1303–8.
Gera M, Saharan VA, Kataria M, Kukkar V. Mechanical method for dry particle coating processes and their applications in drug delivery and development. Recent Pat Drug Deliv Formul. 2010;4:58–81.
Alonso M, Alguacil FJ. Dry mixing and coating of powders. Rev Metal. 1999;35:315–28.
Polizzi M, Langdon BA, Beach L, Mullarney MP. Applying Dry Powder Coatings. Pharm Technol. 2011;212.3:397–402.
Zhou Q, Armstrong B, Larson I, Stewart PJ, Morton DA. Improving powder flow properties of a cohesive lactose monohydrate powder by intensive mechanical dry coating. J Pharm Sci. 2010;99:969–81.
ASTM. Standard test method for shear testing of powders using the Freeman Technology FT4 powder rheometer shear cell. West Conshohocken: ASTM International; 2015.
Hare C, Zafar U, Ghadiri M, Freeman T, Clayton J, Murtagh MJ. Analysis of the dynamics of the FT4 powder rheometer. Powder Technol. 2015;285:123–7.
Freeman Technology, W7006 The conditioning process, 2006.
Freeman Technology, W7013 Stability and variable flow rate method, 2007.
Freeman Technology, W7030 The basic flowability energy, 2008.
Freeman Technology, W7031 Specific energy, 2008.
Freeman Technology, W7018 Shear cell, 2010.
Leonard G, Abatzoglou N. Stress distribution in lubricated vs unlubricated pharmaceutical powder columns and their container walls during translational and torsional shear testing. Powder Technol. 2010;203:524–47.
Rhodes M. Introduction to particle technology. Hoboken: J. Wiley et Sons, Éds., Wiley; 1998.
Schulze D. Powders and bulk solids: behavior, characterization, storage and flow. Heidelberg: Springer Berlin; 2008.
Enerpac, P-series lightweight hand pumps.
GlobePharma, Manual tablet compaction machine model MTCM-I.
Fu X, Huck D, Makein L, Armstrong B, Willen U, Freeman T. Effect of particle shape and size on flow properties of lactose powders. Particuology. 2012;10:203–8.
Malkowska S, Khan A. Effect of re-compression on the properties of tablets prepared by dry granulation. Drug Dev Ind Pharm. 1983;9:331–47.
Acknowledgements
The authors would like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC), the Fonds de recherche du Quebec–Nature et technologies (FRQNT) and Pfizer for their financial support through the Industrial Innovation Scholarships Program (BMP Innovation). Secondly, the authors would like to thank Hosokawa Micron Powder Systems and more precisely C. C. Huang and M. Cavaliere. Finally, the authors would like to thank Pr. R. Gosselin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hudon, S., Lapointe-Garant, PP., Simard, JS. et al. Evaluation of a Dry Coating Technology as a Substitute for Roller Compaction for Dry Agglomeration Applications in the Pharmaceutical Industry. J Pharm Innov 14, 286–303 (2019). https://doi.org/10.1007/s12247-018-9353-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9353-x