Abstract
Purpose
Ellipta® is a new dry-powder inhaler (DPI), with medium flow resistance. The present study aimed to evaluate Ellipta® dose preparation and inhalation technique and determine the effect of human factor, inhalation flow, and inhalation volume on total emitted dose (TED).
Methods
Two-hundred obstructive lung disease patients were asked to load Ellipta® dose and inhale from placebo Ellipta®, without receiving counseling (first attempt). They were divided into patients who previously used DPI (100 patients) and others who never used DPI before (100 patients). Secondly, TED of single-loaded dose from Relvar-Ellipta® was determined at different inhalation flows (20, 40, and 60 L/min) and inhalation volumes (2 and 4 L). TED was also determined after loading the dose twice at inhalation flows of 40 and 60 L/min and inhalation volume of 4 L. Doses were prepared while Ellipta® is in upright and horizontal positions.
Results
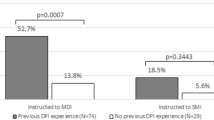
The number of handling errors performed by patients who previously used DPI was lower compared to others who never used DPI before. No significant difference was found between TEDs of 40 and 60 L/min inhalation flow at 2 or 4 L inhalation volume when loading Ellipta®, once or twice, in an upright or horizontal position. TED at inhalation flow of 20 L/min was significantly lower than at 40 and 60 L/min (p < 0.001). A 4-L inhalation volume significantly increased TED than 2 L/min only at inhalation flow of 20 L/min (p = 0.001).
Conclusions
Ellipta® is a consistent DPI. It can be used to deliver inhaled medication at a flow of ≥ 40 L/min without fear of not receiving the needed dose. It does not allow delivering double dosing.

Similar content being viewed by others
References
Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckland, NZ). 2015;8:131.
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
Abdelrahim M, Assi K, Chrystyn H. Dose emission and aerodynamic characterization of the terbutaline sulphate dose emitted from a Turbuhaler at low inhalation flow. Pharm Dev Technol. 2013;18(4):944–9.
Ali AMA, Abdelrahim MEA. Modeling and optimization of terbutaline emitted from a dry powder inhaler and influence on systemic bioavailability using data mining technology. J Pharm Innov. 2014;9(1):38–47.
Abdelrahim ME. Emitted dose and lung deposition of inhaled terbutaline from Turbuhaler at different conditions. Respir Med. 2010;104(5):682–9.
Chapman KR, Fogarty CM, Peckitt C, Lassen C, Jadayel D, Dederichs J, et al. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6(1):353–63.
Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, Broeders M, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604.
Newman S, Busse W. Evolution of dry powder inhaler design, formulation, and performance. Respir Med. 2002;96(5):293–304.
Tarsin W, Assi KH, Chrystyn H. In-vitro intra- and inter-inhaler flow rate-dependent dosage emission from a combination of budesonide and eformoterol in a dry powder inhaler. J Aerosol Med. 2004;17(1):25–32.
von Schantz S, Katajavuori N, Antikainen O, Juppo A. Evaluation of dry powder inhalers with a focus on ease of use and user preference in inhaler-naïve individuals. Int J Pharm. 2016;509(1):50–8.
Yun Kirby S, Zhu C-Q, Kerwin EM, Stanford RH, Georges G. A preference study of two placebo dry powder inhalers in adults with COPD: Ellipta® dry powder inhaler (DPI) versus DISKUS® DPI. COPD: J Chron Obstruct Pulmon Dis. 2016;13(2):167–75.
Hamilton M, Leggett R, Pang C, Charles S, Gillett B, Prime D. In vitro dosing performance of the ELLIPTA® dry powder inhaler using asthma and COPD patient inhalation profiles replicated with the electronic lung (eLung™). J Aerosol Med Pulm Drug Deliv. 2015;28(6):498–506.
Komase Y, Asako A, Kobayashi A, Sharma R. Ease-of-use preference for the ELLIPTA® dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naïve Japanese volunteers aged 40 years or older. Int J Chron Obstruct Pulmon Dis. 2014;9:1365.
Grant AC, Walker R, Hamilton M, Garrill K. The ELLIPTA® dry powder inhaler: design, functionality, in vitro dosing performance and critical task compliance by patients and caregivers. J Aerosol Med Pulm Drug Deliv. 2015;28(6):474–85.
GSK. What makes the ELLIPTA inhaler easy to use? 2018 [cited 2018 10/08/2018]. Available from: https://www.gsksource.com/pharma/content/gsk/source/us/en/brands/breo-asthma/pi/ellipta-inhaler.html.
Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration. 2008;75(1):18–25.
Nicola M, Elberry AA, Sayed OM, Hussein RRS, Abdelrahim MEA. Effect of DPI’s training-device on inhalation technique and clinical efficacy in asthmatics. BJBAS. 2018;7(2):178–83.
Elgendy MO, Abdelrahim ME, Salah Eldin R. Potential benefit of repeated MDI inhalation technique counselling for patients with asthma. Eur J Hosp Pharm. 2015;22:318–22.
Elgendy MO, Abdelrahim ME, Eldin RS. Potential benefit of repeated dry powder inhaler’s inhalation technique counseling on asthmatic patients. Pulm Ther. 2015;1(1):91–101.
Masimukku SK, Chintala R. Development and validation of stability indicating RP-HPLC method for fluticasone furoate and vilanterol in pharmaceutical formulations. Indian Drugs. 2018;55(3):27–31.
Nicola M, Elberry A, Sayed O, Hussein R, Saeed H, Abdelrahim M. The impact of adding a training device to familiar counselling on inhalation technique and pulmonary function of asthmatics. Adv Ther. 2018;35:1049–58.
Chrystyn H. The Diskus Inhaler. Clin Drug Investig. 1999;18(4):287–96.
Boshra MS, Almeldien AG, Salah Eldin R, Elberry AA, Abdelwahab NS, Salem MN, et al. Total emitted dose of salbutamol sulphate at different inhalation flows and inhalation volumes through different types of dry powder inhalers. Exp Lung Res. 2018; (in press).
Al-Showair RA, Pearson SB, Chrystyn H. The potential of a 2Tone Trainer to help patients use their metered-dose inhalers. Chest. 2007;131(6):1776–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeed, H., Salem, H.F., Rabea, H. et al. Effect of Human Error, Inhalation Flow, and Inhalation Volume on Dose Delivery from Ellipta® Dry-Powder Inhaler. J Pharm Innov 14, 239–244 (2019). https://doi.org/10.1007/s12247-018-9352-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9352-y