Abstract
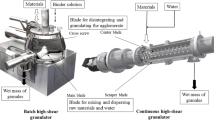
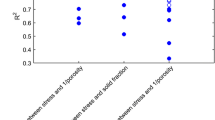
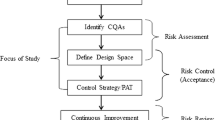
Continuous tablet manufacturing has been investigated for its potential advantages (e.g., cost, efficiency, and controllability) over more conventional batch processes. One avenue for tablet manufacturing involves roller compaction followed by milling to form compactible granules. A better understanding of these powder processes is needed to implement Quality by Design in pharmaceutical manufacturing. In this study, ribbons of microcrystalline cellulose were produced by roller compaction and milled in a conical screen mill. A full factorial experiment was performed to evaluate the effects of ribbon density, screen size, and impeller speed on the product size distribution and steady-state mass holdup of the mill. A population balance model was developed to simulate the milling process, and a parameter estimation technique was used to calibrate the model with a subset of experimental data. The calibrated model was then simulated at other processing conditions and compared with additional unused experimental data. Statistical analyses of the results showed good agreement, demonstrating the model’s predictive capability in quantifying milled product critical quality attributes within the experimental design space. This approach can be used to optimize the design space of the process, enabling Quality by Design.












Similar content being viewed by others
References
Barrasso D, Ramachandran R. A comparison of model order reduction techniques for a four-dimensional population balance model describing multi-component wet granulation processes. Chem Eng Sci. 2012;80:380–2.
Biggs C, Sanders C, Scott A, Willemse A, Hoffman A, Instone T, Salman A, Hounslow M. Coupling granule properties and granulation rates in high-shear granulation. Powder Technol. 2003;130(13):162–8.
Bilgili E, Scarlett B. Population balance modeling of nonlinear effects in milling processes. Powder Technol. 2005;153(1):59–71.
Boukouvala F, Dubey A, Vanarase A, Ramachandran R, Muzzio FJ, Ierapetritou M. Computational approaches for studying the granular dynamics of continuous blending processes, 2 Population balance and data-based methods. Macromol Mater Eng. 2012;297(1):9–19.
Braumann A, Kraft M, Mort PR. Parameter estimation in a multidimensional granulation model. Powder Technol. 2010;197(3):196–210.
Byers JE, Peck GE. The effect of mill variables on a granulation milling process. Drug Dev Ind Pharm. 1990;16(11):176–79.
Cameron I, Wang F, Immanuel C, Stepanek F. Process systems modelling and applications in granulation: a review. Chem Eng Sci. 2005;60(14):3723–50.
Chaudhury A, Kapadia A, Prakash AV, Barrasso D, Ramachandran R. An extended cell-average technique for multi-dimensional population balance models describing aggregation and breakage. Adv Powder Technol. 2013. doi:10.1016/j.apt.2013.01.006.
Immanuel CD, Doyle III FJ. Solution technique for a multi-dimensional population balance model describing granulation processes. Powder Technol. 2005;156(23):213–25.
Iveson SM. Limitations of one-dimensional population balance models of wet granulation processes. Powder Technol. 2002;124(3):219–29.
Kumar J, Peglow M, Warnecke G, Heinrich S, Morl L. Improved accuracy and convergence of discretized population balance for aggregation: the cell average technique. Chem Eng Sci. 2006;61(10):3327–42.
Lionberger RA, Lee S, Lee L, Raw A, Yu L. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268–76.
Motzi JJ, Anderson NR. The quantitative evaluation of a granulation milling process II. Effect of output screen size, mill speed and impeller shape. Drug Dev Ind Pharm. 1984;10(5):713–28.
Murugesu B. Milling. In: Augsburger L, Hoag S, editors . Pharmaceutical dosage forms: unit operations and mechanical properties, 3rd ed; 2008, p. 175–93.
Muzzio FJ, Shinbrot T, Glasser BJ. Powder technology in the pharmaceutical industry: the need to catch up fast. Powder Technol. 2002;124(1):1–7.
Pandya J, Spielman L. Floc breakage in agitated suspensions: effect of agitation rate. Chem Eng Sci. 1983;38(12):1983–92.
Plumb K. Continuous processing in the pharmaceutical industry: changing the mind set. Chem Eng Res Des. 2005;83(6):730–8.
Poon JM-H, Immanuel CD, Doyle III FJ, Litster JD. A three-dimensional population balance model of granulation with a mechanistic representation of the nucleation and aggregation phenomena. Chem Eng Sci. 2008;63(5):1315–29.
Poon JM-H, Ramachandran R, Sanders CF, Glaser T, Immanuel CD, Doyle III FJ, Litster JD, Stepanek F, Wang F-Y, Cameron IT. Experimental validation studies on a multi-dimensional and multi-scale population balance model of batch granulation. Chem Eng Sci. 2009;64(4):775–86.
Process Systems Enterprise Ltd. 2012. gPROMS Advanced User Guide. Vol. 3.6.
Ramachandran R, Barton PI. Effective parameter estimation within a multi-dimensional population balance model framework. Chem Eng Sci. 2010;65(16):4884–93.
Ramkrishna D. Chapter 4 - The solution of population balance equations. In: Population balances. San Diego: Academic; 2000, pp. 117–95.
Samanta A, Ng K, Heng P. Cone milling of compacted flakes: process parameter selection by adopting the minimal fines approach. Int J Pharm. 2012;422:17–23.
Schaber SD, Gerogiorgis DI, Ramachandran R, Evans JMB, Barton PI, Trout BL. Economic analysis of integrated continuous and batch pharmaceutical manufacturing: a case study. Ind Eng Chem Res. 2011;50(17):10083–92.
Schenck L, Plank R. Impact milling of pharmaceutical agglomerates in the wet and dry states. Int J Pharm. 2008;348:18–26.
Sen M, Ramachandran R. A multi-dimensional population balance model approach to continuous powder mixing processes. Adv Powder Technol. 2013;24(1):51–59.
Singh R, Boukouvala F, Jayjock E, Ramachandran R, Ierapetritou M, Muzzio F. Flexible multipurpose continuous processing. Pharm Process. 2012;27(6):22–25.
Vanarase A, Aslam R, Oka S, Muzzio F. Effects of mill design and process parameters in milling of ceramic (alumina-magnesia) extrudates. 2012. In preparation.
Vendola T, Hancock B. The effect of mill type on two dry-granulated placebo formulations. Pharm Technol. 2008;32:72–86.
Wilburn KR. The business case for continuous manufacturing of pharmaceuticals. Master’s thesis, Massachusetts Institute of Technology; 2010.
Yu L. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25:781–91.
Acknowledgments
This work is supported by the National Science Foundation Engineering Research Center on Structured Organic Particulate Systems Grant NSF-ECC 0540855. The authors would also like to thank their industrial mentors: Nishanth Gopinathan and Brian Anderson (Abbott); Mehdrad Langroudi and Jean Hacherl (Merck); Dan Blackwood (Pfizer); Joe Zhou, John Chlapik, and Patrick Putman (Lilly); Dongmei Quiang (Boehringer Ingelheim); Vishwas Nesarikar and Xiaodong Chen (Bristol-Myers Squibb); Mike Brandley (GlaxoSmithKline); Kevin Bittorf and Marco Verwijs (Vertex); and Pieter Schmal (Process Systems Enterprise).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrasso, D., Oka, S., Muliadi, A. et al. Population Balance Model Validation and Predictionof CQAs for Continuous Milling Processes: toward QbDin Pharmaceutical Drug Product Manufacturing. J Pharm Innov 8, 147–162 (2013). https://doi.org/10.1007/s12247-013-9155-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-013-9155-0