Abstract
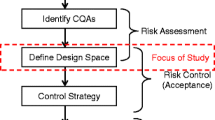
This paper describes the development of an orthogonal design space for a compression-mix blending unit operation for the manufacture of tablet dosage form using an empirical approach. Potential critical process parameters identified through a risk assessment process were assessed through a full-factorial design of experiment for impact on material attributes and drug product critical quality attributes (DP CQA). The impact on each individual attribute measured as responses were subjected to statistical analysis by analysis of variance and regression models were built on the statistically significant effects (p < 0.05). Design space for relevant DP CQA was created using 95% predicted interval estimates. Orthogonal design space for the unit operation was proposed by overlaying design spaces generated for individual DP CQAs. The resulting orthogonal design space made implementation of manufacturing flexibility in to routine manufacturing process and into control strategy simpler and straightforward.










Similar content being viewed by others
References
International Conference on Harmonization of Technical Requirements of Pharmaceuticals for Human Use, ICH Harmonized Tripartite Guideline, Pharmaceutical Development Q8, Step 4, 10-November-2005.
Guidance for Industry, Q8 (R2) Pharmaceutical Development Revision 2, US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), November 2009.
Lepore J, Spavins J. PQLI design space. J Pharm Innov. 2008;3:79–87.
Ragnarsson G, Hölzer AW, Sjögren J. The influence of mixing time and colloidal silica on the lubricating properties of magnesium stearate. Int J Pharm. 1979;3:127–31.
Acknowledgments
The authors greatly acknowledge and thank Lindsay Wylie for the review and useful input on the manuscript and Nathaniel Schuster for his help and support with analytical testing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapsi, S.G., Castro, L.D., Muller, F.X. et al. Development of a Design Space for a Unit Operation: Illustration Using Compression-Mix Blending Process for the Manufacture of a Tablet Dosage Form. J Pharm Innov 7, 19–29 (2012). https://doi.org/10.1007/s12247-012-9122-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-012-9122-1