Abstract
Nitrogen loading from anthropogenic sources, including fertilizer, manure, and sewage effluents, has been linked with declining water quality in coastal lagoons worldwide. Freshwater inputs to mid-Atlantic coastal lagoons of the USA are from terrestrially influenced sources: groundwater and overland flow via streams and agricultural ditches, with occasional precipitation events. Stable nitrogen isotopes ratios (δ15N) in bioindicator species combined with conventional water quality monitoring were used to assess nitrogen sources and provide insights into their origins. Water quality data revealed that nutrients derived from terrestrial sources increased after precipitation events. Tissues from two bioindicator species, a macroalgae (Gracilaria sp.) and the eastern oyster (Crassostrea virginica) were analyzed for δ15N to determine spatial and temporal patterns of nitrogen sources. A broad-scale survey assessment of deployed macroalgae (June 2004) detected regions of elevated δ15N. Macroalgal δ15N (7.33 ± 1.15‰ in May 2006 and 6.76 ± 1.15‰ in July 2006) responded quickly to sustained June 2006 nutrient pulse, but did not detect spatial patterns at the fine scale. Oyster δ15N (8.51 ± 0.89‰) responded slowly over longer time periods and exhibited a slight gradient at the finer spatial scale. Overall, elevated δ15N values in macroalgae and oysters were used to infer that human and animal wastes were important nitrogen sources in some areas of Maryland’s coastal bays. Different nitrogen integration periods across multiple organisms may be used to indicate nitrogen sources at various spatial and temporal scales, which will help focus nutrient management.
Similar content being viewed by others
Introduction
Physical, chemical, and biological indicators are routinely used for monitoring the spatial and temporal extent of eutrophication. However, monitoring eutrophication symptoms does not identify origins of the causative nutrients. In addition, chemical indicators commonly used to measure eutrophication (e.g., total nitrogen or total phosphorus; Nixon 1995; Cloern 2001; Kemp et al. 2005; Bricker et al. 2008) do not detect biologically incorporated nitrogen (Costanzo et al. 2001). Analyzing stable nitrogen isotopes (δ15N) in bioindicator species can be used to address these limitations, as the approach has been shown to identify sources of human and animal wastes (Costanzo et al. 2001; Cohen and Fong 2005). Standard water chemistry measurements of eutrophic symptoms can be complemented with δ15N in bioindicator species to increase understanding of the location and potentially infer the sources of nitrogen.
Comparison of δ15N values in bioindicator species has been used to distinguish between chemically synthesized nitrogen fertilizer and human and animal waste sources (McClelland and Valiela 1998). Fertilizer production fixes atmospheric N2 (defined as 0‰), and as a result, nitrogen runoff from agricultural areas potentially has lower values of δ15N. Human and animal wastes entering groundwater have δ15N values that are elevated (e.g., Sweeny and Kaplan 1980; Tucker et al. 1999) due to a combination of volatilization of ammonia and denitrification, which leave the remaining nitrogen pool enriched with 15N (McClelland and Valiela 1998; Fry 2006). Many wastewater treatment plants employ microbial processing to remove nitrogen at rates higher than in natural ecosystems. Microbial nitrogen removal processes, particularly denitrification, favor the isotopically light 14N and enrich the remaining nitrate pool with 15N (Cline and Kaplan 1975; Kendall 1998). Additionally, ammonia from human and animal waste fractionates during volatilization, leaving the non-volatile portion further enriched with 15N (McClelland and Valiela 1998, Fry 2006). Since multiple processes enrich δ15N values in biological indicator species, interpretations need to be balanced against a set of alternative hypotheses. Measurements of δ15N in biological indicator species are advantageous over direct measurements that can be made on groundwater (Aravena et al. 1993), the water column, or sediments (Tucker et al. 1999), as biota minimize temporal and spatial variability. In particular, this study focused on nitrogen incorporated into macroalgae and filter feeders.
Integration of nitrogen sources occurs over different timescales in different organisms (Gartner et al. 2002; Dattagupta et al. 2004). While δ15N integration in diets has been examined across taxonomic groups (including mollusks) and diets (Vanderklift and Ponsard 2003), the temporal integration of δ15N over various timescales by different organisms, due to species-specific turnover rates, has not been fully explored. Macroalgae uptake nitrogen directly from the water column and have rapid nitrogen turnover rates and so can provide information about available nitrogen over a period of days (Costanzo et al. 2001). Assuming nitrogen limitation, fractionation during assimilation will be minimal (Fry 2006). Oysters are sessile, euryhaline filter feeders that derive nitrogen from a variety of sources, e.g., microorganisms, phytoplankton, detritus, and inorganic particles (Langdon and Newell 1996), and have tissue nitrogen turnover rates in the order of weeks to months depending on the tissue type (Moore 2003). Temporal integration of nitrogen suggested that δ15N in zebra mussels was appropriate to monitor watershed development and downstream effects despite seasonal variations (Fry and Allen 2003). Feeding over multiple trophic levels in field studies may complicate interpretation of δ15N, which is enriched 3–4‰ over each trophic level (Fry 2006). In certain cases, spatial gradients in δ15N could reflect variability in available diets. Nevertheless, biological indicators such as macroalgae and oysters allow an assortment of questions to be addressed through manipulative field experiments that provide long-term integration on different timescales, which is missed by water chemistry measurements alone.
Multiple sources of anthropogenic nitrogen affect mid-Atlantic coastal bays. Collectively, agricultural fertilizers as well as human and animal wastes have been directly linked to downstream eutrophication (Kennish 2002; Kiddon et al. 2003; Bricker et al. 2008; Wazniak et al. 2007). Long-term water quality monitoring reported recent degradation and increases in total nitrogen despite historical improvements and decreases in total nitrogen, signaling a need to better understand the driving forces for trend shifts in this region and identify sources of anthropogenic nitrogen (Wazniak et al. 2007). Symptoms of degradation include an approximate doubling of dissolved organic nitrogen, increasing frequency of harmful algal blooms, e.g., brown tide (Glibert et al. 2007), and adverse effects on seagrass distribution and density (Harris et al. 2005; Wazniak et al. 2007). Human population in Maryland’s coastal bays watersheds doubled between 1980 and 2000 to ~35,000 people and is expected to double again by 2020 (Hager 1996). Septic and wastewater nitrogen inputs have also increased during this period (MCBP 2005). Identifying and differentiating sources of anthropogenic nitrogen can help target management efforts to reduce inputs.
This paper develops a framework for interpreting δ15N from macroalgae (Gracilaria sp.) and oyster (Crassostrea virginica) tissue by addressing three questions: (1) What are the relative capabilities of macroalgae and oysters to detect nitrogen from human and animal wastes? (2) What are the broad-scale spatial patterns of nitrogen from wastes spanning these coastal bays (~600 km2)? (3) What are the fine-scale spatial patterns of influence by nitrogen from human and animal wastes within regions (ranging from ~10 to 50 km2) of Maryland’s coastal bays?
Methods
Study Location
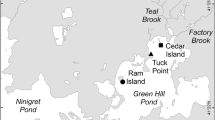
This study was conducted in a series of coastal lagoons located on the mid-Atlantic coast of the USA (Fig. 1). These coastal lagoons, including Chincoteague Bay (extending from 38°15′14″ N, 75°11′57″ W in the north to 37°54′14″ N, 75°24′38″ W in the south), cover the full length of Maryland’s and some of Virginia’s Atlantic coastline. The bays comprise a series of shallow (2-m mean depth), well-mixed, lagoonal estuaries behind barrier islands (Fenwick and Assateague Islands).
Geographical reference of Maryland’s coastal bays within the Delmarva peninsula. In 2004, macroalgae was deployed at 248 sites (triangles) across Maryland’s coastal bays (a). The 2006 deployment of macroalgae and oyster (circles) spanned 100 randomly distributed sites across four regions of interest: b St. Martin River, c Public Landing, d Johnson Bay, and e southern Chincoteague Bay
Due to small watershed areas (totaling 452 km2) of Maryland’s coastal bays, freshwater inputs and activities that result in anthropogenic nitrogen inputs generally occur within 6 km of shore as compared to larger ecosystems (e.g., Jordan et al. 1997; Brawley et al. 2000; Turner and Rabalais 2003). Freshwater base flow, transporting nitrate from terrestrial recharge areas, enters Delmarva Peninsula’s coastal lagoons via both groundwater (Andres 1992; Bratton et al. 2004; Krantz et al. 2004; Manheim et al. 2004) and overland sources that include riverine (Lung 1994; Schwartz 2003) and agricultural ditches (Schmidt et al. 2007). Seasonal precipitation is variable across these coastal bays (Fig. 2). Salinities range from fresh in some tributaries to polyhaline (30–35‰) in the bays. There is oceanic flushing through two small channels: one near Ocean City (38°19′31″ N, 75°05′33″ W) toward the northern end of the bays and the other south of Chincoteague Bay (37°52′36″ N, 75°25′04″ W; Fig. 1). Flushing rates are around 12 days in St. Martin River and 63 days in Chincoteague Bay (Pritchard 1960; Lung 1994). Land cover in the watersheds of these coastal lagoons is dominated by forests (39.5%) and crop agriculture (31.8%), although industrial poultry feeding operations (1.1%) are also located within the watersheds (Table 1). The region has a high occurrence of septic systems for the residential towns of Berlin, MD and Chincoteague, VA (Souza et al. 1993). Poor water quality has been reported in the northern portion of Chincoteague Bay, which is the receiving waters for the town of Berlin, MD (Boynton 1993; Boynton et al. 1996).
Experimental Design
Macroalgae were used for both broad- and fine-scale surveys. Macroalgae (Gracilaria sp.) were deployed at 248 randomly distributed sites throughout all regions of Maryland’s coastal bays from 7 to 12 June 2004 (Fig. 1). Finer scale surveys were conducted from 22 to 27 May 2006 and again from 13 to 18 July 2006. Survey dates were not selected a priori for association with precipitation events, yet 12 precipitation events occurred between fine-scale surveys in June 2006 (0.3–53.3 mm; Fig. 2). During the finer scale surveys, macroalgae was deployed at 100 sites randomly distributed across these coastal bays in St. Martin River (21 sites), Chincoteague Bay at Public Landing (22 sites), Johnson Bay (28 sites), and southern Chincoteague Bay (29 sites; see Fig. 1).
Macroalgae surveys followed the deployment methods described by Costanzo et al. (2001). The macroalgae used for deployment were initially collected in Greenbackville, VA near southern Chincoteague Bay 1 day in advance of deployment. Three subsamples (~1.0-g dry weight each) provided an initial δ15N value (10.0 ± 0.1‰ in June 2004, 5.2 ± 0.2‰ in May 2006, and 9.5 ± 0.6‰ in July 2006). The remaining macroalgae were subsampled (~1.0-g dry weight) for deployment and placed in transparent perforated (35 holes of ~1.0-cm diameter distributed across the side and bottom) containers (130 mL) to allow light, water, and nutrient exchange. For each site, containers (one per site) were attached to anchored surface buoys at a depth of 0.5 of the Secchi depth (rounded to nearest 10 cm).
Oysters (C. virginica) were deployed in the fine-scale survey (2006) in a similar manner as macroalgae. Oysters were originally hatchery-reared without shell substrate (cultchless) <1 year old (29.8- to 95.8-mm shell height) and grown in two locations in St. Martin River (8.2 ± 0.3‰). Oysters were deployed in Johnson Bay and St. Martin River from 21 May to 13 July 2006 and in southern Chincoteague Bay and Public Landing from 22 May to 14 July 2006. Oyster deployments overlapped the June precipitation events. Three oysters from a randomly selected growth location were placed in a mesh (1.9-cm holes) cage, anchored by bricks, and suspended 0.5 m above bottom by surface buoys. The oysters were deployed at the same 100 sites as the macroalgae (Fig. 1).
Data Collection and Analysis
After the deployment period, tissues from both macroalgae and oysters were analyzed for stable isotope ratios (δ15N and δ13C). Upon collection, samples were kept on ice in the field and frozen at the laboratory (−20°C) until processing. Of the surviving oysters from each site, one was selected at random and dissected to recover the adductor muscle for δ15N analysis. Tissues from both organisms were thawed, rinsed, and oven-dried at 60°C for 48 h or until thoroughly dry. Dried macroalgae tissue was finely ground using a grinding mill (Crescent 3110B Wig-L-Bug), while a mortar and pestle was used for oysters. Subsamples (2.0 ± 0.2-mg dry weight of macroalgae, 1.0 ± 0.2-mg dry weight of oyster) were placed in tin capsules (pressed, standard weight 8 × 5 mm, elemental microanalysis). Nitrogen and carbon content (μg N and μg C) and natural abundance of stable isotopes (δ15N and δ13C) were analyzed at the University of California Davis Stable Isotope Facility using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). Molecular %N and C/N ratio were calculated. Both δ15N and δ13C = (R sample/R standard − 1) × 103, where R was defined as either the 15N/14N or 13C/12C ratio. The standard reference was atmospheric N2 (air), with 0.3663 at.% 15N, defined as 0‰ (e.g., Fry 2006), while PDB standard was used for δ13C.
Data on physical parameters and nutrient concentrations were collected and analyzed in conjunction with biological data. Physical (e.g., temperature and salinity) parameters were measured with a WTW Multi 197i water quality probe, and Secchi depth was also recorded. Water samples (20 mL) for nutrient analyses (total nitrogen and total phosphorus) were collected and kept on ice in the field 21 May 2006 (before precipitation events) and 13 July 2006 (after precipitation events) until freezing (−20°C) at the laboratory for analysis. Total nutrients, rather than inorganic species, were analyzed according to standard methods (D’Elia et al. 1977; Kerouel and Aminot 1987). Long-term nitrogen increases and recycling in these bays have been driven by the dominant dissolved organic fraction (Glibert et al. 2001; Glibert et al. 2007) and locally are at least moderately bioavailable (Seitzinger and Sanders 1999; Seitzinger et al. 2002; Mulholland et al. 2004; Glibert et al. 2006; Wiegner et al. 2006). In culture, Gracilaria cornea efficiently grows on organic (urea) or inorganic (NH4 +, NO3 −, or NO3NH4) nitrogen (Navarro-Angulo and Robledo 1999). Therefore total, rather than dissolved inorganic, nutrients were deemed a better indicator of relative nutrient availability. Water samples (60 mL) for chlorophyll a were filtered onto GF/F filter paper (25-mm diameter) in the field and kept on ice until freezing (−20°C) at the laboratory until spectrophotometric analysis, which was conducted according to standard methods (Arar 1997). Data from two statistical outliers (defined as >±3σ from mean, verified by Grubb’s test) were removed. Precipitation data were collected by National Park Service, Assateague Island National Seashore. Spatial patterns for all parameters were plotted with ArcMap 8.0 geographical information system. The Spatial Analyst functionality of the ArcGIS package was used to Krige raster interpolations for measured variables and their variances. If spatial autocorrelation was not confirmed, the interpolation was removed. Correlations were calculated for physical and nutrient parameters for both months. Assumptions of normality and homogeneity of variances were verified with SAS 9.1.2 (Proc Univariate) and no data transformations were required. Statistical analysis testing for differences between means using two-way analyses of variance (ANOVAs; region, month) was also performed with SAS 9.1.2 (Proc Mixed) for all parameters, except for those involving oysters. Oyster data were analyzed with one-way ANOVAs run on regions since only one deployment was conducted, from May to July.
Physical (including nutrients) and biological (chlorophyll a, macroalgae %N, macroalgae δ15N, macroalgae δ13C, and macroalgae C/N) parameters were analyzed with non-metric multidimensional scaling (non-metric MDS) to assess spatial and temporal patterns. Separate analyses were conducted on range standardized physical/nutrient metrics and on biological metrics for each month. A Bray–Curtis similarity matrix produced a distance matrix for each set of variables, which was ordinated by non-metric MDS using PATN (Belbin 1993). Each analysis was conducted in two dimensions with ten random starts. Ordinations had acceptable (0.14 and 0.17, respectively) stress levels (Clarke and Warwick 1994).
Results
Freshwater Inputs Pulsed Nutrients to the Shallow Lagoons
Freshwater inputs were variable across the study period and altered salinity and nutrient concentrations. In 2004, precipitation was consistently low (0.0–15.0 mm) in the spring months (March to May) preceding the broad scale survey. However, there were 12 precipitation events in June 2006 (0.3–53.3 mm; Fig. 2). While total nitrogen was positively correlated with temperature, both total nitrogen and total phosphorus were negatively correlated with salinity (Table 2). Salinity decreased from May 2006 (30.1) to July 2006 (27.7), as precipitation induced a pulse of runoff and diluted the bays. Salinity decreased towards shore at Johnson Bay and upstream at St. Martin River, while salinities at Public Landing and Chincoteague were more homogenous (Fig. 3a–d). Higher concentrations of water column total nitrogen and total phosphorus were found in July 2006 (51.6 ± 15.8 μM N, 4.42 ± 1.04 μM P) than May 2006 (44.6 ± 3.7 μM N, 2.59 ± 0.77 μM P), except for total nitrogen at Johnson Bay. Interpolation of total nitrogen indicated a gradient decreasing offshore (Fig. 3e–h).
Spatial patterns of freshwater and total nitrogen moving offshore were observed between May and July 2006. Salinities are reported for St. Martin River (a), Public Landing (b), Johnson Bay (c), and southern Chincoteague Bay (d). Total nitrogen is reported for St. Martin River (e), Public Landing (f), Johnson Bay (g), and southern Chincoteague Bay (h)
Both temporal and regional differences were found in biological parameters in 2006. Chlorophyll a in Chincoteague and St. Martin River increased with total nitrogen and total phosphorus temporally (Table 3). Yet all variables had a significant interaction between region and month (Table 4). Nutrients pulsed by precipitation events were also incorporated into macroalgae. Macroalgae %N increased from May (1.5%) to July (2.2%). Non-metric MDS indicated biological parameters grouped temporally, but not regionally (Fig. 4a). Chlorophyll a was inversely related to macroalgae δ15N and δ13C values and was not related to macroalgae %N or C/N (Fig. 4b). Macroalgae δ13C was more enriched in July than in May in all regions (Table 3).
Non-parametric multidimensional scaling plot for biological parameters (total chlorophyll a (the sum of chlorophyll a and phaeophytin), macroalgae %N, macroalgae C/N, macroalgae δ15N values, and macroalgae δ13C values (a). Principal axis correlation plot for biological parameters (b). Non-parametric multidimensional scaling plot for physical parameters (Secchi depth, temperature, salinity, total nitrogen, and total phosphorus) (c). Principal axis correlation plot for physical parameters (d)
Broad Spatial Scale Comparisons (2004)
The broad survey in 2004 showed distinct spatial patterns of total nitrogen concentrations across Maryland’s coastal bays. Nutrient concentrations were highest in small creeks and lowest closest to the channels, where bay water exchanges with oceanic water (Fig. 5a). Concentrations of total nitrogen were lowest (0.7 to 38.8 μM) in Isle of Wight Bay, by the channel near Ocean City. Southern Chincoteague Bay, near the other channel, also tended to have low concentrations of total nitrogen (15.8 to 35.0 μM) compared to Public Landing and Johnson Bay (41.2 to 68.8 μM). St. Martin also exhibited moderate concentrations of total nitrogen (42.7 to 72.2 μM). Highest values of total nitrogen were found in Newport Bay (53.7 to 82.1 μM; Fig. 5a). Total nitrogen and total phosphorus concentrations correlated positively with temperature and salinity, but inversely with macroalgal δ15N values (Table 2).
Macroalgal δ15N and %N values varied broadly in 2004 across Maryland’s coastal bays, and spatial patterns differed from that of total nitrogen concentrations. Highest δ15N values were found in southern Chincoteague Bay (10.8‰ to 26.4‰) and then in St. Martin River (12.1‰ to 22.6‰), but were moderate in Public Landing (12.4‰ to 13.2‰). While macroalgal δ15N values in Johnson Bay were moderate (9.5‰ to 17.8‰), the higher values tended to lie to the west of islands in Chincoteague Bay (Fig. 5b). Broad spatial patterns of macroalgae %N were similar to that of δ15N. Macroalgae %N was high in Sinepuxent and Newport Bays in addition to St. Martin River and Isle of Wight Bay. Both macroalgae δ15N and %N were low in Chincoteague Bay, though somewhat elevated around Chincoteague Island (Fig. 5c). Macroalgae %N negatively correlated to total nitrogen (−0.15, p < 0.03) and total phosphorus (−0.35, p < 0.01; Table 2). Spatial patterns of total nitrogen concentrations, macroalgae δ15N, and macroalgae %N did not match (Fig. 5a–c). In St. Martin River, total nitrogen concentrations (55.6 ± 3.0 μM), macroalgae δ15N (15.9 ± 1.2‰), and macroalgae %N (1.5 ± 0.1%) were elevated, but in southern Chincoteague Bay, total nitrogen concentrations (24.1 ± 1.3 μM) and macroalgae %N (1.2 ± 0.1%) were low, while macroalgae δ15N was elevated (17.3 ± 1.3‰).
Fine Spatial Scale Comparisons (2006)
Regional variations in total nitrogen concentrations were detectable in the finer spatial scale sampling data (Fig. 3e–h). During both fine-scale samplings in 2006, St. Martin River had the highest total nitrogen (54.6 ± 1.2 μM N in May, 71.3 ± 3.6 μM N in July) and Johnson Bay had the highest total phosphorus (3.25 ± 0.10 μM P in May, 5.14 ± 0.17 μM P in July), while southern Chincoteague Bay had the lowest total nitrogen (24.8 ± 1.2 μM N in May, 33.9 ± 0.6 μM N in July) and total phosphorus (1.62 ± 0.05 μM P in May, 3.26 ± 0.05 μM P in July; Table 3). Non-metric MDS showed that physical parameters grouped regionally and that total nitrogen and total phosphorus were inversely correlated with Secchi depth (Fig. 4c, d). Southern Chincoteague Bay tended to have low total nutrients and increased Secchi depth, while St. Martin River and Public Landing exhibited gradients of nutrients (Fig. 3e–h). While spatial patterns of macroalgal δ15N were recognizable at the broad scale, they were undetectable at the finer spatial scale within regions in both May and July. The range of macroalgal δ15N values was bigger at the broad spatial scale in 2004 (8.9‰ to 26.4‰) than at the finer spatial scale in 2006 (5.5‰ to 8.8‰ in May and 2.5‰ to 9.1‰ in July). A slight north–south gradient of oyster δ15N emerged within these regions (Fig. 6a, b), particularly at Johnson Bay (7.8‰ to 10.3‰) and southern Chincoteague Bay (6.5‰ to 10.0‰).
Bioindicator Species Comparison
Macroalgae and oyster biological indicators both responded, but in different ways, to nutrient concentrations and sources. At the broad scale (2004), macroalgae δ15N values and total nutrients (total nitrogen and total phosphorus) were inversely related (Table 2). At the finer spatial scale (2006), neither total nitrogen nor total phosphorus significantly related to macroalgae δ15N (Table 2). Absolute change in macroalgae δ15N from initial values were greatest in June 2004 and were negative in July 2006, while changes in oyster δ15N were much smaller than macroalgae, often <±1.0‰ (Fig. 7a). Meanwhile, macroalgae %N decreased from initial values in June 2004 and changed minimally from initial values in either May or July 2006, while oyster %N values exhibited the greatest absolute change in %N, often >1.5% (Fig. 7b). Except in Chincoteague, macroalgae %N was higher in July than May, while macroalgae δ15N decreased from May to July (Table 3). Macroalgae δ15N and %N were positively correlated in May (0.26, p < 0.01, n = 95), but not in July (−0.14, p = 0.17, n = 99). Oyster %N values varied only slightly regionally and exhibited the least increase (1.4%) above initial values at southern Chincoteague Bay. Oyster tissue %N values were spatially consistent with total nitrogen (Table 3). Initial values of macroalgae δ15N (5.2‰ in May and 9.5‰ in July) and oyster δ15N (8.2‰) were lower than final measurements after deployment, except at southern Chincoteague Bay (8.1 ± 0.2‰). Spatially, oyster δ15N values more closely resembled macroalgae δ15N in May 2006 (Johnson Bay > St. Martin River > Public Landing > southern Chincoteague Bay) than those of July 2006. Overall, both macroalgae and oysters had high isotopic values inshore.
Discussion
Freshwater Inputs Pulsed Nutrients to the Shallow Lagoons
Freshwater inputs in June 2006 pulsed nutrients into the coastal lagoons, resulting in changes to the macroalgae nutrient status and phytoplankton abundance. Salinity and nutrient gradients along St. Martin River (Fig. 3a, e) in conjunction with salinity decreases and spatial patterns of nutrients in Public Landing (Fig. 3b, f) and Johnson Bay (Fig. 3c, g) which emanated from shore implicated transport of total nitrogen from terrestrial sources, either via groundwater or overland flow through streams or agricultural ditches. Similar nutrient pulses (e.g., dissolved nitrate) are common in other comparable coastal ecosystems (Valiela et al. 1990; Ullman et al. 2002). Temporal grouping of biological parameters (total chlorophyll a and macroalgae %N, C/N, δ13C, and δ15N) in the non-metric multidimensional scaling analysis indicated that the biological response to nutrients was driven by precipitation (Fig. 4a, b). For example, enrichment of macroalgae δ13C occurred across all regions after June 2006 precipitation events (Table 3). Typical of shallow coastal ecosystems, nutrients increased primary production, which contributed to reduced water clarity via elevated levels of phytoplankton (Fig. 4c, d; Nixon et al. 2001). Macroalgae nitrogen incorporation was inferred from increased %N after freshwater inputs (Table 3).
Patterns at Broad Spatial Scale Identified by Macroalgae
Across these coastal lagoons, total nitrogen concentrations and macroalgae δ15N values provided different information. Spatial patterns of total nitrogen concentrations (Fig. 5a) reflected physical ecosystem processes such as oceanic exchange, as concentrations were low near the two inlets near both ends of Assateague Island and higher in areas with poor flushing, such as Johnson Bay. Elevated macroalgae δ15N in St. Martin River and southern Chincoteague Bay potentially indicated nitrogen sources, possibly from human or animal wastes, even though the concentrations of total nitrogen varied (Fig. 5a, b). This highlights the ability to interpret measurements of δ15N in bioindicators as inputs of human or animal wastes even when total nitrogen concentrations are low. Experimental evidence, such as that with the macroalgae Enteromorpha, suggests that δ15N values are independent of total nitrogen concentration, though the rate of 15N incorporation varies by the form of inorganic nitrogen (Cohen and Fong 2005).
Isotopic values can provide evidence of nitrogen source, particularly in conjunction with land use. Macroalgae were enriched with δ15N in areas where land uses suggested a possible presence of septic and manure sources of nitrogen. Examples include St. Martin River (17.2% residentially developed watershed largely reliant on septic systems) and the adjacent Assawoman Bay (18.9% residentially developed watershed, Table 1). These results align with quantitative linkages that have been made between urban development and enriched δ15N values in primary consumers (Vander Zanden et al. 2005). Animal agriculture, with isotopically enriched manure byproducts, was another comparatively prominent land use feature of these regions (1.8% and 1.4%, respectively, Table 1). St. Martin River, the region with the highest total nitrogen, exhibited a gradient decreasing downstream, suggesting terrestrial nitrogen inputs which diluted upon mixing with higher salinity water from ocean exchange. Yet macroalgae δ15N values in these regions were elevated in the broad (June 2004) survey (Fig. 5b) and in the fine-scale survey prior to rain events (May 2006). While a total nitrogen concentration gradient suggested terrestrially derived nitrogen inputs, septic and/or manure sources were inferred to be important nitrogen sources for St. Martin River and Isle of Wight based upon enriched macroalgae δ15N values.
The town of Chincoteague, VA (population 4,317; 173.1 people per kilometer, US Census Bureau 2000) is situated atop sandy soils and potentially contributes nitrogen via septic systems, as evidenced by enriched δ15N values in macroalgae in the surrounding estuarine waters. This town comprises much of the residential development (1.5% of the total watershed area) in the Chincoteague Bay watershed and relies entirely on septic systems. In addition to elevated δ15N, increased concentrations of total nitrogen would be expected from this potential nitrogen source; however, southern Chincoteague Bay had the lowest total nitrogen at both broad (Fig. 5a) and fine spatial scales (Fig. 3h), likely due to small watershed size, expansive and intact wetlands, and physical processes including dilution and ocean flushing (Wazniak et al. 2007).
Fine Spatial Scale Potentially Indicates Sources Despite Lack of Spatial Patterns
At the fine spatial scale, patterns emerged from oyster δ15N values, but not from macroalgae δ15N values. North–south gradient patterns in Johnson Bay (Fig. 6a) and southern Chincoteague Bay (Fig. 6b) were detectable in oyster muscle δ15N. These gradients agreed with broad patterns from June 2004 macroalgae δ15N (Fig. 5b). Though oyster muscle δ15N gradients were slight, homogeneously elevated values implicated septic sources of nitrogen in southern Chincoteague Bay, likely from the town of Chincoteague, VA. In a similar study, spatial homogeneity of elevated δ15N among hard clam tissues (Mercenaria mercenaria) along a eutrophication gradient has been attributed to anthropogenic sources, suggesting that elevated δ15N in mollusks can still indicate nitrogen source despite a lack of spatial pattern (Oczkowski et al. 2008). Furthermore, oyster δ15N values in this study were similar, though somewhat lower (8.5 ± 0.1‰), to muscle tissue (9.4 ± 0.2‰ and 16.0 ± 2.3‰) of an Australian oyster species (Saccostrea glomerata) influenced by wastewater treatment effluent within 50 m (Piola et al. 2006).
Bioindicator Species Comparison
The growing literature on biological indicators suggests that δ15N in organisms sampled from natural communities consisting of various taxonomic groups, including macrophytes (e.g., McClelland et al. 1997; Cole et al. 2004; Cohen and Fong 2006), finfish (Lake et al. 2001), and mollusks (Fila et al. 2001; McKinney et al. 2002; Vander Zanden et al. 2005), or some combination (Gartner et al. 2002; Fry et al. 2003) can identify nitrogen sources. Additionally, manipulative deployment of macroalgae has been used to interpolate spatial patterns in anthropogenic sources of nitrogen through experimental field work (e.g., Udy and Dennison 1997; Costanzo et al. 2001). The current study combines the benefits of each technique, providing direct comparison between taxonomic groups of primary producers and consumers along with the ability to interpolate spatial patterns based on a manipulative field design in areas where natural communities may not be currently or readily available.
The presence or absence of spatial patterns in δ15N in macroalgae and oysters at different spatial scales provides a spatial context in which each can be usefully deployed as a biological indicator. While clear spatial patterns in macroalgae δ15N and %N emerged at the broad spatial scale in June 2004 (Fig. 5b, c), spatial patterns in δ15 N or %N were not obvious for macroalgae deployed at the fine spatial scale (either May or July 2006). Macroalgae δ15N values were homogenously <10‰ throughout Johnson Bay and southern Chincoteague Bay in both May and July 2006. Therefore, macroalgae may potentially be more usefully deployed as biological indicators of nitrogen source at a broad scale (100 s of km2) rather than at the fine spatial scale (10 s of km2). The slight gradients in Johnson Bay (Fig. 6a) and southern Chincoteague Bay (Fig. 6b) suggest that oyster δ15N may indicate potential nitrogen source at fine spatial scales (10 s of km2).
In this study, manipulative deployments of macroalgae over multiple years in conjunction with deployment of oysters provided a comparison of isotopic responses to water chemistry factors (i.e., the nutrient pulse) in addition to the comparison between species. Macroalgae δ15N and %N exhibited smaller changes from initial values after receiving more precipitation during 2006 in the fine-scale survey than the drier 2004 broad-scale survey (Fig. 7a, b). Decreased regional mean macroalgal δ15N values with increased standard errors from May to July 2006 (Table 3) and the undefined spatial patterns in Johnson Bay can be explained by a relatively short nitrogen turnover rate. Quick turnover rates in macroalgae result in rapid response by δ15N to environmental conditions as compared to slower tissue turnover rates in tissues of consumers such as oysters (Moore 2003; Cohen and Fong 2005). Similar to other studies (e.g., Gartner et al. 2002 and Fry et al. 2003), responsiveness to nitrogen cycling, as reflected in changes to δ15N and %N, was greater in macroalgae than in oysters (Fig. 7a, b), likely due to relative physiological turnover times, days for macroalgae and weeks for oysters. Between regions of these coastal lagoons, patterns of oyster tissue δ15N values (July 2006; Table 3) were more similar to previous macroalgae δ15N values (May 2006; Table 3) than to concurrent macroalgae δ15N values (July 2006; Table 3) and did not reflect short-term nutrient pulses from the June 2006 precipitation events.
Interpretations of δ15N in macroalgae and oysters to infer nitrogen source may be influenced by water chemistry factors as well as the spatial scale of interest. Isotopic signals can be influenced by physical conditions such as salinity, temperature, or depth (Jennings and Warr 2003) and are variable in strength. Water chemistry measurements varied over time in these coastal bays, as evidenced by a wide range of macroalgae δ15N values in 2004 (Fig. 5b) and a smaller range of macroalgae δ15N values with no clear finer scale spatial pattern in 2006. Since spatial patterns were detectable in 2006 by oyster δ15N, perhaps these tissues are less susceptible to variability in water chemistry due to oyster physiology (Fig. 7a, b).
A combination of indicator species responsiveness and ecosystem features may affect the success of indicating nitrogen source. For example, oceanic mixing and short residence times in deep waters offshore southwestern Australia may have dispersed δ15N signals before transmission from organic sources to filter feeders via food sources, though more responsive macroalgae reflected sewage effluent sources (Gartner et al. 2002). In another study in the northeast Atlantic, Jennings and Warr (2003) found that most spatial δ15N variability in scallops is related to physical conditions (salinity, depth, and temperature). Comparatively, the shallow coastal lagoons of the present study are characterized by residence times on the order of weeks (Pritchard 1960; Lung 1994); potentially enough time for δ15N signals to persist and be incorporated into oysters, provided a sufficient time period for oyster uptake and assimilation.
Variation in responsiveness based on physiological differences between primary producers and filter feeders may potentially introduce a lag time in oyster δ15N values compared those of macroalgae. The greater absolute changes found in macroalgae δ15N compared to oyster δ15N (Fig. 7a) suggest a more rapid response to nitrogen source, likely due to relative physiological turnover times. For example, when comparing across regions, oyster δ15N values (July 2006) more closely resemble macroalgae δ15N values from May 2006 (Johnson Bay > St. Martin River > Public Landing > southern Chincoteague Bay) than macroalgae δ15N values from July 2006 (Table 3). This discrepancy between macroalgal and oyster δ15N values may have been magnified by a time lag due to different rates or modes of nitrogen assimilation. Macroalgae assimilate nitrogen directly from the water column (e.g., Cohen and Fong 2006), while oysters receive their nitrogen indirectly from the water column (via consumption of a variety of nitrogen sources, for example microorganisms, phytoplankton, detritus and inorganic particles) to reflect ambient δ15N (Newell and Langdon 1996; Cohen and Fong 2005). Due to the rate and timing of nitrogen assimilation, oysters integrate nitrogen in the muscle over longer time periods than macroalgae (Moore 2003). Future studies could investigate the possibility of a lag time in oyster δ15N response as compared to macroalgae due to variations in length of nitrogen incorporation.
In addition to differences between macroalgae and oysters, different species within a functional group may provide different temporal integrations based on species-specific turnover rates. Muscle tissues in different species of filter feeding bivalves vary, e.g., ~2 months for the eastern oyster (C. virginica), >3 months for Sydney rock oyster (Saccostrea commericalis; Moore 2003), and >1 year for a methanotrophic hydrocarbon seep mussel (Bathymodiolus childressi; Dattagupta et al. 2004). Because the diet of filter feeders includes multiple trophic levels (e.g., primary producers, detritus, etc.), which are separated by 2–3‰ (Fry 2006), mixtures of trophic levels may confound interpretation of δ15N. In certain cases, spatial gradients in δ15N could reflect variability in available diets. Yet identifying human and animal waste as potential nitrogen source to these bays fits with recent degradation of water quality and increases in total nitrogen identified by long-term monitoring datasets (Wazniak et al. 2007). Multiple temporal integrations among species allow different monitoring questions to be addressed by different biological indicators. Macroalgae and oysters may be suited for different roles as biological indicators, but they both may have the potential to indicate nitrogen sources at various spatial and temporal scales, which will help focus nutrient management.
References
Andres, A.S. 1992. Estimate of nitrate flux to Rehoboth and Indian River Bays, Delaware, through direct discharge of ground water. Open File Report No. 35, Delaware Geological Survey, Newark DE. 39 pp.
Arar, E.J. 1997. Method 446.0 In vitro determination of chlorophylls a, b, c1 + c 2 and pheopigments in marine and freshwater algae by visible spectrophotometry. Cincinnati, Ohio: Revision 1.2 National Exposure Research Laboratory Office of Research and Development, U.S. Environmental Protection Agency.
Aravena, R., M.L. Evans, and J.A. Cherry. 1993. Stable isotopes of oxygen and nitrogen in source identification of nitrate from septic systems. Ground Water 31:180–186. doi:10.1111/j.1745-6584.1993.tb01809.x.
Belbin, L. 1993. PATN pattern analysis package: User’s guide. Commonwealth Scientific and Industrial Research Organisation, Division of Wildlife and ecology.
Boynton, W.R. (ed.). 1993. Maryland’s coastal bays: An assessment of aquatic organisms, pollutant loadings and management options. Chesapeake Biological Laboratory, Solomons, Maryland. Ref. No. [UMCEES] CBL 93-053.
Boynton, W.R., L. Murray, J.D. Hagy, C. Stokes, and W.M. Kemp. 1996. A comparative analysis of eutrophication patterns in a temperate coastal lagoon. Estuaries 19:408–421. doi:10.2307/1352459.
Bratton, J.F., J.K. Böhlke, F.T. Manheim, and D.E. Krantz. 2004. Ground water beneath coastal bays of the Delmarva Peninsula: Ages and nutrients. Ground Water 42:1021–1034. doi:10.1111/j.1745-6584.2004.tb02641.x.
Brawley, J.W., G. Collins, J.N. Kremer, C.H. Sham, and I. Valiela. 2000. A time-dependent model of nitrogen loading to estuaries from coastal watersheds. Journal of Environmental Quality 29:1448–1461.
Bricker, S.B., B. Longstaff, W. Dennison, A. Jones, K. Boicourt, C. Wicks, and J. Woerner. 2008. Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae 8:21–32. doi:10.1016/j.hal.2008.08.028.
Clarke, K.R., and R.M. Warwick. 1994. Change in marine communities: An approach to statistical analysis and interpretation. Plymouth, UK: Natural Environment Research Council.
Cline, J.D., and I.R. Kaplan. 1975. Isotopic fractionation of dissolved nitrate during denitrification in the eastern tropical North Pacific Ocean. Marine Chemistry 3:271–299. doi:10.1016/0304-4203(75)90009-2.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210:223–253. doi:10.3354/meps210223.
Cohen, R.A., and P. Fong. 2005. Experimental evidence supports the use of δ15N content of the opportunistic green macroalga Enteromorpha intestinalis (Chlorophyta) to determine nitrogen sources to estuaries. Journal of Phycology 41:287–293. doi:10.1111/j.1529-8817.2005.04022.x.
Cohen, R.A., and P. Fong. 2006. Using opportunistic green macroalgae as indicators of nitrogen supply and sources to estuaries. Ecological Applications 16:1405–1420. doi:10.1890/1051-0761(2006)016[1405:UOGMAI]2.0.CO;2.
Cole, M.L., I. Valiela, K.D. Kroeger, G.L. Tomasky, J. Cebrian, C. Wigand, R.A. McKinney, S.P. Grady, and M.H. Carvalho da Silva. 2004. Assessment of a δ15N isotopic method to indicate anthropogenic eutrophication in aquatic ecosystems. Journal of Environmental Quality 33:124–132.
Costanzo, S.D., M.J. O’Donohue, W.C. Dennison, N.R. Loneragan, and M. Thomas. 2001. A new approach for detecting and mapping sewage impacts. Marine Pollution Bulletin 42:149–156. doi:10.1016/S0025-326X(00)00125-9.
D’Elia, C.F., P.A. Steudler, and N. Corwin. 1977. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnology and Oceanography 22:760–764.
Dattagupta, S., D.C. Bergquist, E.B. Szalai, S.A. Macko, and C.R. Fisher. 2004. Tissue carbon, nitrogen, and sulfur stable isotope turnover in transplanted Bathymodiolus childressi mussels: Relation to growth and physiological condition. Limnology and Oceanography 49:1144–1151.
Fila, L., R.H. Carmichael, A. Shriver, and I. Valiela. 2001. Stable N isotopic signatures in bay scallop tissue, feces, and pseudofeces in Cape Cod estuaries subject to different N loads. Biological Bulletin 201:294–296. doi:10.2307/1543374.
Fry, B. 2006. Stable isotope ecology. 1st ed. New York, NY: Springer.
Fry, B., and Y.C. Allen. 2003. Stable isotopes in zebra mussels as bioindicators of river-watershed linkages. River Research and Applications 19:683–696. doi:10.1002/rra.715.
Fry, B., A. Gace, and J.W. McClelland. 2003. Chemical indicators of anthropogenic nitrogen-loading in four Pacific estuaries. Pacific Science 57:77–101. doi:10.1353/psc.2003.0004.
Gartner, A., P. Lavery, and A.J. Smit. 2002. Use of δ15N signatures of different functional forms of macroalgae and filter-feeders to reveal temporal and spatial patterns in sewage dispersal. Marine Ecology Progress Series 235:63–73. doi:10.3354/meps235063.
Glibert, P.M., R. Magnien, M.W. Lomas, J. Alexander, C. Fan, E. Haramoto, M. Trice, and T.M. Kana. 2001. Harmful algal blooms in the Chesapeake and coastal bays of Maryland, USA: Comparison of 1997, 1998, and 1999 events. Estuaries 24(6A):875–883. doi:10.2307/1353178.
Glibert, P.M., J. Harrison, C. Heil, and S. Seitzinger. 2006. Escalating worldwide use of urea—A global change contributing to coastal eutrophication. Biogeochemistry 77:441–463. doi:10.1007/s10533-005-3070-5.
Glibert, P.M., C.E. Wazniak, M.R. Hall, and B. Sturgis. 2007. Seasonal and interannual trends in nitrogen and brown tide in Maryland’s coastal bays. Ecological Applications 17(5):S79–S87. doi:10.1890/05-1614.1.
Hager, P. 1996. Worcester County, MD. p. 20–24. In K. Beidler, P. Gant, M. Ramsay, and G. Schultz (eds.), Proceedings—Delmarva’s Coastal Bay watersheds: Not yet up the creek. EPA/600/R-95/052. United States Environmental Protection Agency, National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division, Narragansett, Rhode Island, USA.
Harris, L., S. Granger, and S. Nixon. 2005. Evaluation of the health of eelgrass (Zostera marina L.) beds within the Maryland coastal bays: A report to the National Park Service. Narragansett, RI, USA: University of Rhode Island, Graduate School of Oceanography.
Jennings, S., and K.J. Warr. 2003. Environmental correlates of large-scale spatial variation in the δ15N of marine animalsMarine Biology 142:1131–1140.
Jordan, T.E., D.L. Correll, and D.E. Weller. 1997. Effects of agriculture on discharges of nutrients from coastal plain watersheds of Chesapeake Bay. Journal of Environmental Quality 26:836–848.
Kemp, W.M., W. Boynton, J. Adolf, D. Boesch, W. Boicourt, G. Brush, J. Cornwell, T. Fisher, P. Glibert, J. Hagy, L. Harding, E. Houde, D. Kimmel, W.D. Miller, R.I.E. Newell, M. Roman, E. Smith, and J.C. Stevenson. 2005. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Marine Ecology Progress Series 303:1–29. doi:10.3354/meps303001.
Kendall, C. 1998. Tracing nitrogen sources and cycling in catchments. In Isotope tracers in catchment hydrology, eds. C. Kendall, J.J. cDonnell, and .New York: Elsevier.
Kennish, M.J. 2002. Environmental threats and environmental future of estuaries. Environmental Conservation 29:78–107. doi:10.1017/S0376892902000061.
Kerouel, R., and A. Aminot. 1987. Procédure optimisée hors-contaminations pour l’analyze des éléments nutritifs dissous dans l’eau de mer. Marine Environmental Research 22:19–32. doi:10.1016/0141-1136(87)90079-1.
Kiddon, J.A., J.F. Paul, H.W. Buffam, C.S. Strobel, S.S. Hale, D. Cobb, and B.S. Brown. 2003. Ecological condition of US mid-Atlantic estuaries 1997–1998. Marine Pollution Bulletin 46:1224–1244. doi:10.1016/S0025-326X(03)00322-9.
Krantz, D.E., F.T. Manheim, J.F. Bratton, and D.J. Phelan. 2004. Hydrogeologic setting and ground water flow beneath a section of Indian River Bay, Delaware. Ground Water 42:1035–1051. doi:10.1111/j.1745-6584.2004.tb02642.x.
Lake, J.L., R.A. McKinney, F.A. Osterman, R.J. Pruell, J. Kiddon, S.A. Ryba, and A.D. Libby. 2001. Stable nitrogen isotopes as indicators of anthropogenic activities in small freshwater systems. Canadian Journal of Fisheries and Aquatic Science 58:870–878. doi:10.1139/cjfas-58-5-870.
Langdon, C.J., and R.I.E. Newell. 1996. Digestion and nutrition in larvae and adults, pp. 231–260 In The Eastern Oyster Crassostrea virginica, eds. Kennedy, V.S., Newell, R.I.E. and Eble, A.F. Maryland Sea Grant.
Lung, W.S. 1994. Water quality modeling of the St. Martin River, Assawoman and Isle of Wight Bays. Baltimore, Maryland: Maryland Department of the Environment.
Manheim, F.T., D.E. Krantz, and J.F. Bratton. 2004. Studying ground water under Delmarva Coastal Bays using electrical resistivity. Ground Water 42:1052–1068. doi:10.1111/j.1745-6584.2004.tb02643.x.
MCBP [Maryland Coastal Bays Program]. 2005. About the Maryland Coastal Bays Program. Annapolis, Maryland: Maryland Department of Natural Resources.
McClelland, J.W., and I. Valiela. 1998. Linking nitrogen in estuarine producers to land-derived sources. Limnology and Oceanography 43:577–585.
McClelland, J.W., I. Valiela, and R.H. Michener. 1997. Nitrogen-stable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds. Limnology and Oceanography 42:930–937.
McKinney, R.A., J.L. Lake, M.A. Charpentier, and S. Ryba. 2002. Using mussel isotope ratios to assess anthropogenic nitrogen inputs to freshwater ecosystems. Environmental Monitoring and Assessment 74:167–192. doi:10.1023/A:1013824220299.
Moore, K.B. 2003. Uptake dynamics and sensitivity of oysters to detect sewage-derived nitrogen. BSc Honours Thesis, University of the Sunshine Coast, Sippy Downs, Australia.
Mulholland, M.R., G. Boneillo, and E.C. Minor. 2004. A comparison of N and C uptake during brown tide (Aureococcus anophagefferens) blooms from two coastal bays on the east coast of the USA. Harmful Algae 3:361–376. doi:10.1016/j.hal.2004.06.007.
Navarro-Angulo, L., and D. Robledo. 1999. Effects of nitrogen source, N:P ratio and N-pulse concentration and frequency on the growth of Gracilaria cornea (Gracilariales, Rhodophyta) in culture. Hydrobiologia 398/399:315–320. doi:10.1023/A:1017099321188.
Newell, R.I.E. and C.J. Langdon. 1996. Mechanism and physiology of larval and adult feeding, pp. 185–223. In The Eastern Oyster Crassostrea virginica, eds. Kennedy, V.S., Newell, R.I.E. and Eble, A.F. Maryland Sea Grant.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41:199–219.
Nixon, S.W., B. Buckley, S. Granger, and J. Bintz. 2001. Responses of very shallow marine ecosystems to nutrient enrichment. Human and Ecological Risk Assessment 75:1457–1481. doi:10.1080/20018091095131.
Oczkowski, A., S. Nixon, K. Henry, P. DiMilla, M. Pilson, S. Granger, B. Buckley, C. Thornber, R. McKinney, and J. Chaves. 2008. Distribution and trophic importance of anthropogenic nitrogen in Narragansett Bay: An assessment using stable isotopes. Estuaries and Coasts 31:53–69. doi:10.1007/s12237-008-9102-3.
Piola, R.F., S.K. Moore, and I.M. Suthers. 2006. Carbon and nitrogen stable isotope analysis of three types of oyster tissue in an impacted estuary. Estuarine Coastal and Shelf Science 66:255–266. doi:10.1016/j.ecss.2005.08.013.
Pritchard, D.W. 1960. Salt balance and exchange rate for Chincoteague Bay. Chesapeake Science 1:48–57. doi:10.2307/1350536.
Schmidt, J.P., C.J. Dell, P.A. Vadas, and A.L. Allen. 2007. Nitrogen export from coastal plain field ditches. Journal of Soil and Water Conservation 62:235–243.
Schwartz, M.C. 2003. Significant groundwater input to a coastal plain estuary: Assessment from excess radon. Estuarine Coastal and Shelf Science 56:31–42. doi:10.1016/S0272-7714(02)00118-X.
Seitzinger, S.P., and R.W. Sanders. 1999. Atmospheric inputs of dissolved organic nitrogen stimulate estuarine bacteria and phytoplankton. Limnology and Oceanography 44:721–730.
Seitzinger, S.P., R.W. Sanders, and R.V. Styles. 2002. Bioavailability of DON from natural and anthropogenic sources to estuarine plankton. Limnology and Oceanography 47:353–366.
Souza, S., B. Krinsky, and J. Seibel. 1993. Maryland’s coastal bays: An assessment of aquatic ecosystems, pollutant loadings, and management options. Baltimore, MD: Maryland Department of the Environment.
Sweeny, R.E., and I.R. Kaplan. 1980. Tracing flocculent industrial and domestic sewage transport on San Pedro Shelf, southern California, by nitrogen and sulphur isotope ratios. Marine Environmental Research 3:215–224. doi:10.1016/0141-1136(80)90028-8.
Tucker, J., N. Sheats, A.E. Giblin, C.S. Hopkinson, and J.P. Montoya. 1999. Using stable isotopes to trace sewage-derived material through Boston Harbor and Massachusetts Bay. Marine Environmental Research 48:353–375. doi:10.1016/S0141-1136(99)00069-0.
Turner, R.E., and N.N. Rabalais. 2003. Linking landscape and water quality in the Mississippi River Basin for 200 years. BioScience 53:563–572. doi:10.1641/0006-3568(2003)053[0563:LLAWQI]2.0.CO;2.
Udy, J.W., and W.C. Dennison. 1997. Physiological responses of seagrasses used to identify anthropogenic nutrient inputs. Marine and freshwater research 48:605–614. doi:10.1071/MF97001.
Ullman, W.J., A.S. Andres, J.R. Scudlark, and K.B. Savidge. 2002. Storm-water and base-flow sampling and analysis in the Delaware inland bays: Preliminary report of findings 1998–2000: Open File Report No. 44, Delaware Geological Survey, Newark DE. 44p.
US Census Bureau. 2000. Summary File 1 (SF 1) and Summary File 3 (SF 3).
Valiela, I., J. Costa, K. Foreman, J.M. Teal, B. Howes, and D. Aubrey. 1990. Transport of groundwater-borne nutrients from watersheds and their effects on coastal waters. Biodegradation 10:177–197.
Vander Zanden, M.J., Y. Vadeboncoeur, M.W. Diebel, and E. Jeppesen. 2005. Primary consumer stable nitrogen isotopes as indicators of nutrient source. Environmental Science and Technology 39:7509–7515. doi:10.1021/es050606t.
Vanderklift, M.A., and S. Ponsard. 2003. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 136:169–182. doi:10.1007/s00442-003-1270-z.
Wazniak, C.E., M.R. Hall, T.J.B. Carruthers, B. Sturgis, W.C. Dennison, and R.J. Orth. 2007. Linking water quality to living resources in a Mid-Atlantic lagoon system, USA. Ecological Applications 17(5):S64–S78. doi:10.1890/05-1554.1.
Wiegner, T.N., S.P. Seitzinger, P.M. Glibert, and D.A. Bronk. 2006. Bioavailability of dissolved organic nitrogen and carbon from nine rivers in the eastern United States. Aquatic Microbial Ecology 43:277–287. doi:10.3354/ame043277.
Acknowledgments
Thanks to J. Davis, A. Dungan, M. Hall, J. Schofield, E. Sherry, B. Sturgis, J. Testa, C. Wazniak, and C. Wicks for field and lab assistance, as well as Horn Point Analytical Services and UC Davis Stable Isotope Facility. J. O’Neil provided laboratory space. Thanks also to E. Fertig, T. Fisher, R. Jesien, D. Meritt, R. Newell, B. Sturgis and C. Wazniak for invaluable discussions and insight. Assateague Island National Seashore provided precipitation monitoring data. The authors thank the Maryland Coastal Bays Program and the NOAA OCRM for grant support, and the Maryland Coastal Bays Oyster Gardening Program for oysters. This is UMCES contribution number 4268.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fertig, B., Carruthers, T.J.B., Dennison, W.C. et al. Oyster and Macroalgae Bioindicators Detect Elevated δ15N in Maryland’s Coastal Bays. Estuaries and Coasts 32, 773–786 (2009). https://doi.org/10.1007/s12237-009-9148-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-009-9148-x