Summary
Since its description, almost 100 years ago, the genus Dinizia has been treated as monospecific, comprising the single canopy-emergent species Dinizia excelsa Ducke which grows in non-flooded Amazonian forests of Guyana, Suriname and seven states of northern and central-western Brazil. Dinizia jueirana-facao G. P. Lewis & G. S. Siqueira, which grows in a restricted area of semi-deciduous Atlantic rain forest in Espírito Santo state, Brazil, is described as a new species in the genus. The new species is also a canopy-emergent of impressive stature. We provide descriptions for both species, a key to species identification, a distribution map and the new species is illustrated. Fossil leaves, inflorescences and fruit provide evidence for a Dinizia-like ancestor occurring in south-eastern North America during the Eocene. In contrast to D. excelsa where pollen is dispersed in tetrads, the pollen of D. jueirana-facao is shed in monads. D. jueirana-facao is considered critically endangered following IUCN conservation criteria, whereas D. excelsa is assessed to be of least concern. A lectotype is designated for D. excelsa.
Similar content being viewed by others
Introduction
For almost 100 years the genus Dinizia has been treated as monospecific. The genus was first described by Ducke (1922) to accommodate the single species D. excelsa Ducke, a rain forest canopy-emergent of impressive stature (some individuals over 60 m tall are recorded from the Brazilian Amazon). Ducke named the tree after his friend José Picanço Diniz, doctor-in-law and philanthropist, thanks to whom botanical exploration in Trombetas was made possible. Burkart (1943) placed Dinizia in his tribe Mimozygantheae based on the similar imbricate sepals and indehiscent fruits of D. excelsa and Mimozyganthus carinatus (Griseb.) Burkart. In addition, both species have a nectary in a distinct hypanthium (Ancibor 1969). The fruits of the two species are, however, vastly different in size and texture and we now know that the two species are not closely related phylogenetically (Luckow et al. 2005); the nectary in the hypanthium appears to have evolved independently in the two taxa. The tribe Mimozygantheae has since been disbanded, and Mimozyganthus Burkart was shown to belong to tribe Mimoseae, and sister to a clade comprising the two genera Piptadeniopsis Burkart and Prosopidastrum Burkart (Luckow et al. 2005). Luckow et al. (2003), based on molecular and morphological data, found Dinizia to be more closely related to caesalpinioid genera than to genera in the Mimosoideae. This placement of the genus is supported by it having flowers with a hypanthium, a stylar groove, and imbricate petals, “characters either unusual or unknown among other mimosoids” (Luckow et al. 2003). Indeed, Ducke (1949) had already commented on the apparent intermediate position of Dinizia between the mimosoids and the caesalpinioids. Barneby et al. (2011) excluded Dinizia from their treatment of the mimosoids for the Flora of the Guianas. Recent molecular studies (Bruneau et al. 2008; LPWG 2017) have placed Dinizia in the Caesalpinioideae, close to some other members of the Dimorphandra group. The genus now belongs to a re-circumscribed Caesalpinioideae, but is not closely related to any genera in the mimosoid clade (LPWG 2017).
Fossils
Fossil leaves of Duckeophyllum eocenicum Herendeen & Dilcher (1990), the co-occurring fossil inflorescence, Eomimosoidea plumosa Crepet & Dilcher (1977), and specimens of fossil pods, Eliasofructus catahoulensis Herendeen & Dilcher (1990) and E. claibornensis Herendeen & Dilcher (1990) may well all represent a single extinct genus, and the vegetative and reproductive fossils have each been compared with the extant species Dinizia excelsa (Herendeen & Dilcher 1990). The fossils provide evidence for a Dinizia-like ancestor occurring in south-eastern North America during the Eocene. The fossil flower Eomimosoidea plumosa has pollen in permanent tetrads, further evidence of an association with D. excelsa which also has pollen in tetrads. The fossil fruits are similar to those of D. excelsa in several features. Fruit shape and texture, and especially the presence of longitudinal wrinkles near the fruit margin, are all characteristic of the fossils and of Dinizia excelsa (Herendeen & Dilcher 1990).
A new species supported by molecular analyses
Just over a decade ago, Renato Moraes Jesus, then the biodiversity general manager of the Compania Vale do Rio Doce (CVRD) in Linhares, Espirito Santo, Brazil, sent to Kew an unidentified legume specimen (Folli 4889) taken from a very large tree growing inside the Reserva Natural Vale. The suggestion, based on field characteristics, that this might be a new species of the mimosoid legume genus Parkia R. Br. proved incorrect. Based on inflorescence type, flower morphology and the treeʼs robust woody fruit it seemed more likely that the specimen was related to the caesalpinioid genus Dimorphandra Schott. Years later, after the gathering of more field data, together with molecular, palynological, and morphological studies, it is clear that the tree growing in the Atlantic Forest of the Vale Reserve represents a new, second species of the genus Dinizia. Molecular phylogenetic studies that have included either or both plastid and nuclear DNA sequences for both Dinizia excelsa and Dinizia sp. nov. (in particular samples of Folli 4884, 4889; e.g., Bruneau et al. 2008; Manzanilla & Bruneau 2012; Babineau & Bruneau 2017) find that the two species always group together in a well-supported clade, separate from other clades and species in Caesalpinioideae. Plastid trnL-F, matK and rps16, and nuclear ITS, tRALs and Leafy sequences are available for D. excelsa, and for the new species, plastid trnL-F, matK, rps16, and trnD-T, and nuclear ITS, PP1, tRALs, AIGP, EIF3E and Leafy sequences have been analysed (Bruneau et al. 2008; Manzanilla & Bruneau 2012; LPWG 2017; Babineau & Bruneau 2017). Although the loci sequenced and the taxon sampling differs amongst studies, and although resolution remains poor overall amongst lineages subtending the mimosoid clade in the Caesalpinioideae, all recent molecular phylogenetic analyses suggest that Dinizia occurs in a poorly resolved group that includes Dimorphandra, Campsiandra, Mora, Burkea and Stachyothyrsus, along with Tachigali, Arapatiella and Jacqueshuberia.
Pollen
Guinet (1981), in his seminal contribution to mimosoid legume pollen, stated that, ‘the constant presence of single grains has been found in 14 [mostly species-poor] genera’, while five genera, including Dinizia (the genus was then considered to be a member of the Mimosoideae) have either compound or single grains. Thus, fide Guinet (1981), the genus Dinizia, then represented by the single species D. excelsa, displayed pollen dimorphism, although Guinet published no photographic evidence to support this contention. It is possible that the apparent monads observed by Guinet in D. excelsa were the result of acetolysis breaking the tetrads apart. Nevertheless, the new species of Dinizia has pollen consistently in monads (Fig. 1A & B) and the genus, as now circumscribed here, therefore evidently displays pollen dimorphism.
Dinizia pollen is not the only example of a genus in the legume family that has one species that releases its pollen in tetrads, and another (or others) that releases pollen in monads. Tetrads have arisen at least four times independently in caesalpinioid legumes and are also present in some mimosoid clade legumes. Pollen that is released in tetrads occurs in one or two species of the genera Bauhinia, Diptychandra and Afzelia, with other closely related species being released as normal monads (Sorsa 1969; Ferguson & Banks 1994; Banks 2003; Banks et al. 2010). Relatively minor changes in the timing of exine development during ontogeny, and the presence or absence of callose that surrounds microspores during development, can determine whether microspores remain permanently united in calymmate or acalymmate tetrads when mature, or are released as individual monads (Banks et al. 2010, Lora et al. 2014). The morphology of pollen tetrads varies among the various legume species in which they occur; they are acalymmate in Afzelia, Bauhinia and Dinizia, and calymmate in Diptychandra (Banks & Rudall 2016).
The pollen of Dinizia excelsa analysed by us is in tetrahedral tetrads (Fig, 1C) with the individual grains 3-colporate, perforate, gemmate in the polar areas and clavate in the mesocolpial area (i.e they are covered in wart-like lumps, with the lumps more robust in the mesocolpial areas and less so in the polar areas). Guinet (1981) considered D. excelsa pollen ornamentation to be unique by the occurrence of well-developed clavae mixed with small verrucae. The tetrads are easily differentiated from the single pollen grains of Dinizia sp. nov. (Fig. 1A & B) which have psilate-perforate ornamentation.
Nitrogen fixation
Dinizia excelsa does not nodulate (Moreira et al. 1992; Sprent 2001). Branched structures collected from the roots of Dinizia sp. nov. (Sergio Faria, pers. comm.) are more likely to be ectomycorrhizal than root nodules housing bacteria (J. Sprent, pers. comm.) and it is thus hypothesised that Dinizia sp. nov. also does not nodulate.
Taxonomic account
Herbarium acronyms follow Index Herbariorum (Thiers, continuously updated).
Dinizia Ducke (1922: 76).
Large forest canopy-emergent trees, buttressed or not, bark breaking off in large woody plates. Leaves bipinnate, eglandular, the pinnae alternate to subopposite, leaf and pinnae rachises caniculate along upper margin; leaflets alternate, glabrous or the lower surface puberulent to glabrescent. Inflorescence a compound raceme; flowers hermaphrodite or functionally male, a nectarial ring at the base of the hypanthium surrounding the centrally placed ovary stipe; petals 5, free, imbricate, attached around the upper rim of the hypanthium; the outer surface of the hypanthium, calyx tube and corolla puberulent, the calyx and corolla lobe margins ciliate; stamens 10, free, glabrous, the filaments inserted around the hypanthium rim, anthers eglandular, dorsally fixed; ovary glabrous to pubescent on its lateral faces, style glabrous, stigma terminal, tubular to slightly funnel-shaped. Fruit coriaceous to woody, indehiscent or dehiscent along both sutures, seeds laterally compressed, hard, pleurogram lacking, pollen in monads or tetrads. Type: Dinizia excelsa Ducke.
Key to the species of Dinizia
Leaflets in 7 – 14 pairs per pinna, puberulent to glabrescent on their lower surface; individual inflorescence rachis (10 –) 12.5 – 16 cm long, 1 – 1.5 (– 2) cm wide in open flower; buds ellipsoid to obovoid, the calyx completely covering the petals; bracts lanceolate, persistent to caducous; flowers 4 – 5 mm long; fruits coriaceous, indehiscent, red when immature; seeds (10 –) 14 – 15 × 6 – 7 mm; pollen in tetrads; Amazonian rain forest in Brazil and the Guianas……………………………………… D. excelsa
Leaflets in (9 –) 15 – 24 pairs per pinna, glabrous on both surfaces; inflorescence rachis 28 – 35 cm long, 3 – 4.5 cm wide in open flower; buds globose, the petals exposed early in development; bracts spathulate, caducous; flowers 8.5 – 10 mm long; fruits woody, dehiscent, yellowish cream or greenish when immature; seeds 25 – 30 × 16 – 19 mm; pollen in monads; Atlantic rain forest……………………D. jueirana-facao
Dinizia excelsa Ducke (1922: 76). Type: Brazil, Obidos, Serra do Curumú, 4 Jan. 1914, Ducke s.n. (lectotype MG 15304!, designated here), remaining syntypes: Ducke s.n. (MG nos. 15774, 15826, 15989, 16177, 17073).
A canopy emergent tree, (15 –) 30 – 60 m+, unarmed, trunk cylindrical, bole of larger specimens 15 – 22.5 m, up to 3 m in diam. at soil level, DBH (23 –) 80 cm – 2 m, moderately to strongly buttressed, the buttresses to 4 – 5 m tall (and these “continue off into the forest as raised, laterally compressed roots up to 80 cm high”, Zarucchi et al. 2936), crown spreading; bark smooth, white, breaking off in woody plates to reveal a light red-brown or brick-red under bark; heartwood brown to red, without streaks. Stipules subulate, 3 – 6 mm long, caducous. Leaves bipinnate, eglandular, the petiole terete, 2 – 7.5 cm long, the rachis (4 –) 6 – 28 cm long, caniculate, puberulent; pinnae in 3 – 6 subopposite to strongly alternate pairs, or odd-pinnate with one extra pinna on one side (i.e. total pinnae per leaf 7 to 11 (– 13)), the pinnae 6.5 – 12.5 cm long, the rachis caniculate with raised ridges along each side of the channel, puberulent to pubescent; leaflets alternate, in 7 – 14 pairs per pinna, subsessile, oblong, subelliptic, to trapeziform, 12 – 25 × 5 – 11 mm, leaflet apex retuse to rounded, base truncate, inequilateral about the midvein, the lamina much broader on the distal side of the midvein base, the midvein otherwise subcentral to diagonal, secondary venation brochidodromous, but hardly visible, lamina discolorous, the upper surface darker, glabrous (except for a few hairs on the slightly immersed midvein) and nitid, the lower surface sparsely puberulent to glabrescent, including on the prominent midvein, the margins revolute, the pulvinule fleshy, cone-shaped, puberulent. Inflorescence a multi-branched, terminal compound raceme, its rachis puberulent; individual racemes over 150-flowered, the peduncle 3 – 15 (– 20) mm long, the rachis (8 –) 12.5 – 16 cm long, the raceme 1 – 1.5 (– 2) cm wide in open flower; flowers mostly functionally male (the gynoecium supressed or lacking), fewer flowers in each individual raceme hermaphrodite, all flowers 4 – 5 mm long (from base of pedicel to apex of petals), whitish green to greenish yellow, fragrant, short-pedicellate, the pedicel 0.5 – 1 mm, a persistent to caducous, lanceolate, pubescent, 0.5 mm bract at the base of each flower pedicel, bracteoles lacking, buds globose, the petals exposed early in development; the pedicel, hypanthium, calyx tube and its 5 equal, short, broadly triangular lobes all puberulent with white hairs, the lobe margins ciliate, the hypanthium and calyx tube together 1 – 1.25 mm long; petals 5, free, imbricate, obovate to elliptic, lacking a distinct claw, slightly hooded to dorsally concave, 3 – 4 × 2 – 2.25 mm, sparsely hairy along a central vertical line on the dorsal surface, glabrescent, the margin sparsely to moderately ciliate. Stamens 10, white, 3× petal length, the filaments 10 – 12 mm long, very shortly fused at their bases and attached as a ring to the rim of the hypanthium, anthers uniform, dorsifixed, 0.6 – 0.7 mm, anther glands lacking, staminodes lacking. Gynoecium red, glabrous, the base short-stipitate, style terminating in a slightly flared (funnel-shaped) hollow stigma. Fruit wine-red coloured when fresh (Simon et al. 1452), laterally compressed, coriaceous, glabrous, indehiscent, 20.5 – 35 (including a 1.5 – 2 cm stipe) × 4.5 – 8.5 cm, the sutures longitudinally wrinkled and appearing almost winged, the upper “wing” ± 1 cm wide, 7 – 12-seeded. Seeds oblong to elliptic, sometimes slightly narrower in the middle, (10 –)14 – 15 × 6 – 7 mm, laterally compressed, black, hard (the texture of a pebble), the surfaces with a network of minute fracture lines, pleurogram absent, the apex narrowing to a terminal funicle attachment, < 1 mm. Pollen in acalymmate tetrahedral tetrads with the individual grains 3-colporate, and ornamentation gemmate in the polar areas and clavate in mesocolpial areas (Fig. 1C). Root nodules lacking.
distribution. Guyana, Suriname and Amazonian Brazil (in the northern and central-western states of Amapá, Amazonas, Mato Grosso, Pará, Rondônia, Roraima and Tocantins). Also recorded from the state of Acre by Lorenzi (1992). Map 1.
specimens examined. brazil: Amapá, Serra do Navio, Rio Amapari, trail to Rio Araguary, 2 km from camp, 6 Nov. 1954 (fr.), Cowan 38124 (K!); Mun. de Mazagão, Camaipi, 0°10'N, 51°37'W, 23 Dec. 1984 (fr.), Mori et al. 17511 (K!, NY); Camaipi, c. 0°10'N, 51°37'W, 17 Sept. 1983 (fr.), Mori et al. 16236 (K!, NY); 19 Sept. 1983 (st.), Mori et al. 16385 (K!, NY); 19 Sept. 1983 (st.), Mori et al. 16401 (K!, NY); 19 Sept. 1983 (st.), Mori et al. 16408 (K!, NY); Amazonas, Mun. de Axinim, basin of Rio Abacaxis, lower Rio Paca, 4°07'S, 58°58'W, 1 July 1983 (fr.), Zarucchi et al. 2936 (INPA, K!, NY); Mun. de Manaus, c. 90 km N de Manaus, 02°19'S, 60°05'W, 19 Aug. 1995 (fl.), Nee & Dick 46239 (K!, NY); Manaus, 8 Aug. 1942 (fl.), Ducke 975 (K!); Manaus, Colonia Campos Salles, 27 July 1932 (fl. & fr.), Ducke 24201 (K, 2 sheets!, RB); Manaus, experimental station, km 60, 17 July 1977 (seed only), da Silva 295 (K!); Rio dos Pombos (a tributary of the Yuma river), 74 km E of the Aripuanã river, 21 June 1979 (fl.), Calderón et al. 2646 (INPA, K!); Distrito Agropecuário, 2°24'26" – 2°25'31"S, 59°43'40" – 59°45'50"W, 7 July 1990 (fl.), Mori et al. 21327 (K!, NY); Mato Grosso, Rio Aripuanã, road from Nucleo Pioneiro de Humboldt to Rio Juruena, km 8, 10°12'S, 59°21'W, 25 Oct. 1973 (fr.), Berg & Steward P19869 (K!, NY); Pará, near the Rio Jaburuzinho, 12 July 1923 (buds & fr.), Ducke s.n. (K!, RB No. 16810); Gurupá, 16 May 1916 (buds), Ducke s.n. (RB No. 10240, 2 sheets, barcodes 00539872! and 00547527!, MG No. 16177!); Gurupá, 25 Jan. 1916 (fr.), Ducke s.n. (MG 15982, two sheets!); Rio Tapajoz, região das cachoeiras inferiors (Poção), 26 June 1918 (buds, fr.), Ducke s.n. (RB No. 10241, barcode 00539873!, MG No. 17073); Rio Tapajoz, Bella Vista, 6 Dec. 1915 (fr. & seeds), Ducke s.n. (RB No. 10239, barcode 00539874!, MG No. 15826); Obidos, Serra do Curumú, 1 Oct. 1915 (fr.), Ducke s.n. (MG 15774!); Obidos, Serra do Curumú, 4 Jan. 1914 (fr.), Ducke s.n. (lectotype: MG 15304!); Mt Dourado, Água Azul, 1°7'S, 52°55'W, 4 Jan. 1988 (fr.), Pires & Silva 1907 (K!); Mun. Almeirim, Mt Dourado, 6 July 1987 (fl.), Pires et al. 1713 (K!); 0°40'S, 52°35'W, 20 Jan. 1988 (fr.), Pires & Silva 1955 (K!); 0°47'S, 52°42'W, 31 May 1988 (fr.), Pires & Silva 2172 (K!); Estação Ecol. Jarí, 0°27'S, 52°51'W, 6 Jan. 1988 (fr.), Pires & Silva 1916 (K!); Monte Dourado, 1°03'S, 52°51'W, 8 June 1988 (fr.), Pires & Silva 2214 (K!); Monte Dourado, 00°52'S, 52°33'W, 14 June 1988 (st.), Pires & Silva 2222 (K!); 1°03'S, 52°51'W, 14 July 1988 (buds), Pires 2305 (K!); Rondônia, Porto Velho, ao longo da BR-364, 61 km Leste de Jaci Paraná, ramal 500 m ao Sul, 08°58'17"S, 63°59'16"W, 12 April 2012 (fr.), Simon et al. 1452 (CEN, K 2 sheets!); 09°14'39"S, 64°20'56"W, 14 April 2012 (buds), Simon et al. 1481 (CEN, K!); 09°15'47"S, 64°37'02"W, 15 Aug. 2010 (fr.), Pereira-Silva et al. 15634 (CEN, K!); Mun. de Santa Barbara, rodovia BR-364, km 120, 9°10'S, 63°07'W, 29 May 1982 (fl.), Teixeira et al. 871 (INPA, K!); Roraima, Mun. São João de Baliza, Rio Jatapuzinho, 0°35'N, 59°07'W, Nov. 1994 (fr.), Milliken 2258 (K!). guyana: U.Takutu-U., Essequibo Region, Kamoa Mts, 1°47'22"N, 58°44'18"W, 25 May 1997 (fr.), Clarke 4956 (K!, US); Gunn’s, Essequibo R., 30 Sept. 1989 (fl.), Jansen-Jacobs et al. 1900 (K!, U); Essequibo, Kuyuwini R., 0 – 2 km NW of camp, 02°04'N, 59°17'W, 18 July 1996 (fr.), Clarke 2257 (K!, US); Simuni Creek, Rupununi R., a few miles N of Kanaku Mts, 8 August 1931 (fl.), Davis in Forest Department of British Guiana field no. D128, record no. 2119 (K, 3 sheets!). suriname: Sipaliwini, 3 km S (190°) from Kwamalasamutu village centre, 2°19'30"N, 56°47'20"W, 22 Feb. 2006 (fr.), Hoffman 6691 (K!, US).
habitat. The species clearly prefers non-flooded environments and is recorded from non-inundated moist forest, non-flooded upland mixed forest, “floresta ombrofila mista”, tropical forest on terra firme, tropical upland evergreen forest and tropical dry forest, at elevations from 50 – 490 m.
conservation status. Dinizia excelsa is geographically widely dispersed in seven Brazilian states, Guyana and Suriname and has a tendency to be gregarious (Forest Dept. of British Guiana field no. D128, record no. 2119; and da Silva et al. 1977). The estimated extent of occurrence (EOO) exceeds the thresholds for a threatened category according to IUCN criteria version 3.1 (IUCN 2012) and it is suspected that the area of occupancy (AOO) also exceeds these thresholds. It is therefore assessed as being of Least Concern, although it is not known how frequently encountered the tree is today across its distribution range, and it is evident from the literature that its wood has been widely used (see under notes). The species is not protected under CITES regulations.
phenology. Collected in flower in Brazil from April to August, and in fruit throughout the year (no fruiting collections seen from February or March); in Brazil the main flowering period in most states is July and August; collected in flower in Guyana in August and September and in fruit in May and July, and in Suriname a single fruiting specimen (in K) was collected in February.
common names. “Angelim”, “angelim pedra”, “angelim vermelho”, “paricá” (Brazil); “Awaraimë” (Trio, Suriname); “parakwa” (Wapisiana, Guyana). Lorenzi (1992: 176) also includes the popular names: “angelim falso”, “faveira”, “faveira-dura”, faveira-ferro” and “faveiro-do-grande”.
notes. The species is notable for the hardness of its wood and its bark breaking off in woody plates which accumulate in piles at the base of the tree. Usually the most terminal raceme in the compound inflorescence flowers first, then the basal racemes open, followed by those above; the flowers in each raceme open somewhat irregularly, but generally from the base to the apex of the raceme; freshly opened flowers are strongly fragrant (Nee & Dick 46239). Dinizia excelsa is reported to be pollinated by bees (Ribeiro et al. 1999). The wood is very resistant and difficult to work, but has been widely used for railway sleepers, in civil and naval construction, cabinetwork and joinery (da Silva et al. 1977), and for battens, props, beams, girders, posts, stakes, door and window frames, floor boards, carts, wagons and bridges (Lorenzi 1992). The wood density of D. excelsa is recorded as between 0.83 – 0.91 g/cm3 by Fearnside (1997) and as 0.9 – 1.2 g/cm3 by Richter & Dallwitz (online version 2009), who also describe the wood odour as distinct, very unpleasant and persistent.
In the protologue of Dinizia excelsa, Ducke (1922) cited six specimens that he had collected in Pará between 1914 and 1918 (MG herbarium numbers: 15304, 15774, 15826, 15989, 16177 and 17073) without choosing a holotype. The six collections should thus be considered as syntypes; all are still housed in the Museu Goeldi (MG) herbarium, with some duplicated in the herbarium of the Rio de Janeiro Botanic Gardens (RB). A number of the specimens in MG carry original field labels in Ducke’s handwriting, but no one specimen bears both flower and fruit material. The specimen in the best condition, which fits the description of foliage and fruits presented in the original description, and which includes an original label in Ducke’s hand, is MG15304 from the Serra do Curumú collected on the 4th of January 1914. This specimen is thus designated as the lectotype of D. excelsa. The other specimens cited in the speciesʼ protologue become remaining syntypes.
Dinizia jueirana-facao G. P. Lewis & G. S. Siqueira sp. nov. Type: Brazil, Espírito Santo, Linhares, Reserva Natural Vale, 30 July 2004 (fl.), D. A. Folli 4889 (holotype CVRD!; isotypes HUEFS!, K!).
http://www.ipni.org/urn:lsid:ipni.org:names:60475109-2
Tree, 19 – 40 m, unarmed, trunk 10 – 22 m before first major branching, DBH up to 1.56 m, circumference at breast height (1.40 –) 1.90 – 4.90 m, diam. of crown 10 – 20 m, bark grey, frequently breaking off in large woody plates, sap clear and watery. Stipules not seen. Leaves (including measurements from a 10 year old, 6 m tall, planted tree), alternate to spirally arranged, bipinnate, (25.5 –) 35 – 96 cm long (including the petiole), eglandular; petiole 5.5 – 10 cm long, flattened on upper edge near its base, angular at the margins, ± rounded along lower edge, leaf rachis caniculate along upper margin, the channel becoming more pronounced towards the distal end; pinnae in (9 –) 15 – 19 alternate to sub-opposite pairs per leaf (sometimes an extra terminal pinna on one side of the rachis, the total number of pinnae per leaf thus either even or odd), 9.5 – 15.5 cm long, the proximal and distal pinnae shorter and with fewer leaflet pairs than the median pinnae, the pinna rachis caniculate along its upper edge with pronounced raised puberulent ridges on either side of the channel; leaflets alternate to subopposite, sessile, (9 –) 15 – 23 (– 24) pairs per pinna, their blades sub-rhombiform, coriaceous, 8 – 23 × 2.5 – 6 mm, glabrous on both surfaces, somewhat discolorous on drying, the upper surface darker and shiny, the apex rounded to very shallowly retuse, the base subtruncate to rounded, inequilateral with the distal side of the blade distinctly broader than the proximal side, blade margin entire, slightly thickened, slightly revolute, the midvein ± central, slightly prominent to slightly immersed on the upper surface, distinctly prominent on the lower surface, a fleshy cone-shaped pulvinus at the base of the midvein, secondary venation not visible on the upper surface, brochidodromous but obscure on the lower surface. Inflorescence an erect, terminal, large woody compound raceme, exserted from the surrounding foliage, the racemes in groups of two or more subtended by a woody, 15 – 30 cm long, densely tomentose to puberulent, rust-coloured, primary woody peduncle (the colour contrasting with the white indumentum of the leaf petioles), the individual inflorescence rachis sparsely puberulent, 28 – 35 cm long, 3 – 4.5 cm wide (in open flower), longitudinally ridged (when dry), each raceme with hundreds of pedicellate flowers (fallen flowers leaving narrowly ellipsoid sunken pedicel scars on the rachis, these occasionally filled with a small resin droplet), flowers bright yellow, hermaphrodite (although some apical flowers appearing functionally male due to suppression of gynoecium development), 8.5 – 10 mm long (from base of the robust 1.5 – 2 mm pedicel to the tips of the petals), a caducous, spathulate, stipitate, puberulent, 1.5 – 2 mm bract inserted on an inflorescence ridge directly below each flower pedicel, but these obscure and most evident below buds; calyx valvate in bud, the buds ellipsoid to obovoid, the broadly acute calyx lobes spreading apart in a symmetrical star shape to reveal the petals beneath, calyx of mature flowers campanulate, coriaceous, puberulent on the tube and 5 lobes, the minute white hairs especially dense on the lobe apices, the tubular hypanthium 2 – 2.5 mm long, a darkened nectarial zone at its inner base, the calyx tube c. 2 mm long, the subequal lobes 1.25 – 1.5 mm; petals free, imbricate, 5.5 – 7 × 3 – 3.5 mm, subequal (the median petal the smallest), inserted around the upper margin of the hypanthium, the slightly reflexed blade glabrous on its inner surface, moderately pubescent with white hairs over most of the outer surface, the petal margins densely ciliate, the broad claw almost as wide as the blade; stamens 10, free, c. 20 – 25 mm long, inserted in two whorls (one slightly higher than the other) along the upper margin of the hypanthium, glabrous, anthers uniform, dorsifixed, anther apex with a short thickened connective, anther glands lacking, staminodes lacking; ovary c. 9 mm long, short-stipitate, the stipe 3 – 4 mm, inserted centrally within the hypanthium, pubescent, especially on the two lateral faces, the style glabrous and tapering to an apical, tubular, glabrous stigma. Fruits scimitar-shaped, ± falcate, woody, yellowish cream to greenish when immature, maturing dark brown to black, 40 – 46 × 8.5 – 10 cm, smooth, glabrous, the upper and lower sutures thickened and longitudinally ridged, dehiscent along both sutures, the woody exocarp raised between the seed chambers, 13 – 15-seeded. Seeds black, hard (the texture of pebbles), elliptic to obovate in outline, 25 – 30 × 16 – 19 mm, laterally compressed, sub-nitid along the margins, pleurogram lacking, the lateral surfaces minutely pitted and with a fine network of fracture lines (only visible with a ×10 lens), the apical funicular attachment point 2 – 4 mm long. Pollen oblate, tricolporate, with psilate aperture membranes, c. 30 μm in diam., with psilate-microperforate ornamentation, the mesocolpial areas smooth, the areas around the aperture margins more rugulate and with a higher density of perforations, the aperture margins project over the endoaperture areas, the apices of the apertures fork into indentations that almost join around the apocolpium to form a weakly syncolporate pattern. Root nodules lacking. Figs 1A & B, 2, 3 & 4.
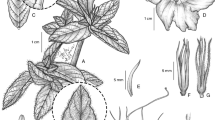
Dinizia jueirana-facao. A flowering branch and part of a bipinnate leaf; B leaflets at the base of a single pinna; C hermaphrodite flower; D functionally male flower opened to show stamen filaments and suppressed gynoecium development; E calyx opened out, outer surface; F longitudinal section of hermaphrodite flower to show gynoecium; G petal, outer surface; H stamen; J anther; K fruit; L part of a single valve of dehisced fruit with seeds attached; M seed. A – J from Folli 4889 (K), K – M from Folli 4484 (K). drawn by margaret tebbs.
recognition. Dinizia jueirana-facao differs from its sister species D. excelsa in having leaflets in (9 –) 15 – 23 (– 24) pairs per pinna (vs 7 – 14 pairs), the leaflets completely glabrous (vs puberulent to glabrescent on their lower surface), its individual racemes 28 – 35 × 3 – 4.5 cm (vs 10 – 18 × 1 – 2 cm), buds ellipsoid to obovoid (vs globose), flowers 8.5 – 10 mm long (vs 4 – 5 mm long), its floral bracts spathulate and caducous (vs lanceolate and often persistent), its fruit woody and dehiscent along both sutures (vs indehiscent), seeds 25 – 30 × 16 – 19 mm (vs (10 –) 14 – 15 × 6 – 7 mm); and pollen in monads (vs tetrads).
distribution. Dinizia jueirana-facao is currently known only from two locations, one (19°08'52.0"S, 40°05'16.4"W) in the Reserva Natural Vale in Linhares, northern Espirito Santo state, Brazil, and the second (19°05'12.1"S, 40°10'41.2"W) just outside the reserve in the surroundings of the small hamlet of Santa Luzia Sooretama. Map 1.
specimens examined. brazil: Espírito Santo: Linhares, Reserva Natural Vale, 20 March 2003 (fr.), Folli 4484 (CVRD!, HUEFS!, K!); 30 July 2004 (fl.), Folli 4888 (CVRD 8816!, HUEFS!); 30 July 2004 (fl.), Folli 4889 (holotype CVRD 8814!, isotypes HUEFS!, K!); Sooretama, UTM 37606, 7889162, 8 Oct. 2014 (fl.), Folli 7270 (CVRD 15119!, RB!); Sooretama, 28 Sept. 2015 (fr.), Folli 7409 (CVRD 15506!); Reserva Natural Vale, 19°08'50"S, 40°05'12"W, 21 May 2013 (st., 6 m sapling tree), Neves et al. 1220 (RB 574861) (BHCB, E!, HUEFS, K!).
habitat. An emergent tree in semi-deciduous forest and mata ciliar in the Reserva Natural Vale, an area of 22,000 hectares of pristine Atlantic Forest. This is the largest protected area of semi-deciduous forest in eastern Brazil. Also known from mata de tabuleiro, in the surroundings of Sooretama, just outside the Vale Reserve. Growing at elevations of 40 – 150 m above sea level.
conservation status. There are two localities of Dinizia jueirana-facao, one within the Reserva Natural Vale and one just outside it, in the surroundings of a small settlement known as Santa Luzia Sooretama, Espirito Santo. In the Reserve only 12 adult trees are known, these distributed across an area of 42.99 hectares (UTM: 385596, 7882465). The locality outside the Reserve also has between ten and 12 trees, these dispersed over an area of 64.81 hectares (UTM: 376061, 7889162). To date, the species is only known from these two small areas, which together contain less than 25 adult trees. The species, especially outside the Reserva Natural Vale, is threatened by habitat loss as a consequence of deforestation due to urban development, agriculture, livestock farming and mining. Although one of the localities is inside a protected area, this is owned by the private mining company Vale and if the company was ever to fall on hard times the reserve could lose its protection. The species is assessed as Critically Endangered (C2a(i,ii)+D) according to IUCN criteria version 3.1 (IUCN 2012), due to its very small and restricted population, combined with an inferred continuing decline in the number of mature individuals based on its habitat deforestation rates.
phenology. Flowering and fruiting times are poorly known and considered to be unpredictable. Collected in flower in July and October and in fruit in March, July and September. It is assumed that the large woody fruits take many months to reach full maturity.
etymology. The species name is taken directly from the local name, “jueirana-facão”, for the tree in Espirito Santo. In the Reserva Natural Vale, the large legume tree Parkia pendula (Willd.) Benth ex Walp. is known as jueirana-vermelha and the new Dinizia species, which has a very similar bark which breaks off in large woody plates, but much larger fruits, is locally differentiated by replacing vermelha (Portuguese for red) with facão (Portuguese for large knife or machete), because the woody fruits of D. jueirana-facao have the appearance of a machete sheath or scabbard. According to the International Code of Nomenclature for algae, fungi and plants (McNeill et al. 2012) an epithet can be a word in apposition (Art. 23.1) and taken from any source whatsoever (Art. 23.2), but the Code does not give clear guidance on diacritical signs, just ruling (Art. 60.6) that “the [diacritical] signs are to be suppressed with the necessary transcription of the letters so modified” but without elaborating on what “necessary transcription” means beyond the cited examples, which do not include ã. We thus transcribe the ã as a in the specific epithet here chosen for the new species.
Jueirana is thought to be derived from the Tupi word yuá-rana. Yuá (or Juá) is a Tupi common name for several different plant species, especially those in the Solanaceae with round, spiny fruits (Andrade 2006; Sampaio 1987). Rana in Tupi means similar to, so yuá-rana or jueirana means false juá (or similar to juá), although there is little resemblance between the new legume species and any Solanaceae. A number of place names in Brazil are derived from jueirana or an orthographic variant of this.
notes . Dinizia jueirana-facao, as currently known, is a narrowly restricted species endemic to a small area of Atlantic forest in the Brazilian state of Espirito Santo. Although a tree of shorter stature, and lacking buttresses, many of its vegetative and reproductive morphological characteristics are greater in number and/or size than those seen in its widespread Amazonian sister species, D. excelsa. D. jueirana-facao has leaflets in (9 –) 15 – 23 (– 24) pairs per pinna (7 – 14 pairs per pinna in D. excelsa), the leaflets glabrous (vs puberulent to glabrescent on their lower surface), its individual racemes 28 – 35 × 3 – 4.5 cm (vs 10 – 18 × 1 – 2 cm) in open flower, its flower buds ellipsoid to obovoid (vs globose), its flowers 8.5 – 10 mm long (vs 4 – 5 mm long), its floral bracts spathulate and caducous (vs lanceolate and often persistent), its fruit woody and dehiscent along both sutures (vs indehiscent), its seeds 25 – 30 × 16 – 19 mm (vs (10 –) 14 – 15 × 6 – 7 mm), and its pollen in monads (vs tetrads). D. jueirana-facao is critically endangered and presently known from less than 25 trees in two small areas, of which only one locality is inside a protected reserve. The type collection of the new species is from one of the largest trees growing inside the reserve.
References
Ancibor, E. (1969). Los nectarios florales en Leguminosas-Mimosóideas. Darwiniana 15: 128 – 140.
Andrade, K. S. (2006). Atlas toponímico de origem indígena do estado do Tocantins — projeto ATITO (PhD thesis). Faculdade de Filosofia, Letras e Ciências Humanas, Universidade de São Paulo, São Paulo.
Babineau, M. & Bruneau, A. (2017). Phylogenetic and biogeographical history of the Afro-Madagascan genera Delonix, Colvillea and Lemuropisum (Fabaceae: Caesalpinioideae). Bot. J. Linn. Soc. 184: 59 – 78. https://doi.org/10.1093/botlinnean/box009
Banks, H. (2003). Structure of pollen apertures in the Detarieae sensu stricto (Leguminosae: Caesalpinioideae), with particular reference to underlying structures (Zwischenkörper). Ann. Bot. 92: 425 – 435.
____, Himanen, I. & Lewis, G. P. (2010). Evolution of pollen, stigmas and ovule numbers at the caesalpinioid-mimosoid interface (Fabaceae). Bot. J. Linn. Soc. 162: 594 – 615.
____ & Rudall, P. (2016). Pollen structure and function in caesalpinioid legumes. Amer. J. Bot. 103: 423 – 536.
Barneby, R. C., Grimes, J. W. & Poncy, O. (2011). Leguminosae subfamily 87. Mimosoideae. In: M. J. Jansen-Jacobs (ed.), Fl. Guianas, Series A, Phanerogams Fascicle 28. Royal Botanic Gardens, Kew.
Bruneau, A., Mercure, M., Lewis, G. P. & Herendeen, P. S. (2008). Phylogenetic patterns and diversification in caesalpinioid legumes. Botany 86: 697 – 718.
Burkart, A. (1943). Las leguminosas argentinas, silvestres y cultivadas. Acme Agency, Buenos Aires.
Crepet, W. L. & Dilcher, D. L. (1977). Investigations of angiosperms from the Eocene of southeastern North America: a mimosoid inflorescence. Amer. J. Bot. 64: 714 – 725.
Da Silva, M. F., Lisbôa, P. L. B. & Lisbôa, R. C. L. (1977). Nomes vulgares de plantas amazônicas. INPA, Belém.
Ducke, A. (1922). Plantes nouvelles ou peu connues de la region amazonienne (IIe Partie). Arch. Jard. Bot. Rio de Janeiro 3: 76 – 78 (Dinizia).
____ (1949). Notas sôbre a Flora Neotrópica–II, As Leguminosas da Amazônia Brasileira. Bol. Técn. Inst. Agron. N. 18: 64 – 65 (Dinizia).
Fearnside, P. M. (1997). Wood density for estimating forest biomass in Brazilian Amazonia. Forest Ecology and Management 90: 59 – 87.
Ferguson, I. K. & Banks, H. (1994). Tetrad pollen in the subfamily Caesalpinioideae (Leguminosae) and its significance. Rev. Palaeobot. Palynol. 83: 31 – 42.
Guinet, Ph. (1981). Mimosoideae: the characters of their Pollen Grains. In: R. M. Polhill & P. H. Raven (eds), Advances in Legume Systematics, part 2: 835 – 857. Royal Botanic Gardens, Kew.
Herendeen, P. S. & Dilcher, D. L. (1990). Fossil mimosoid legumes from the Eocene and Oligocene of southeastern North America. Rev. Palaeobot. Palynol. 62: 339 – 361.
IUCN (2012). IUCN Red List Categories and Criteria: Version 3.1. Second Edition. IUCN, Gland and Cambridge.
Lora, J., Herrero, M. & Hormaza, J. I. (2014). Microspore development in Annona (Annonaceae): differences between monad and tetrad pollen. Amer. J. Bot. 101: 1 – 11.
Lorenzi, H. (1992). Árvores Brasileiras, Manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Editora Plantarum Ltda., Nova Odessa. p. 176 (Dinizia excelsa).
LPWG (2017). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66(1): 44 – 77.
Luckow, M., Fortunato, R. H., Sede, S. & Livshultz, T. (2005). The phylogenetic affinities of two mysterious monotypic mimosoids from southern South America. Syst. Bot. 30(3): 585 – 602.
____, Miller, J. T., Murphy, D. J. & Livshultz, T. (2003). A phylogenetic analysis of the Mimosoideae (Leguminosae) based on chloroplast DNA sequence data. In: B. Klitgaard & A. Bruneau (eds), Advances in Legume Systematics. Part 10, Higher Level Systematics, pp. 197 – 220. Royal Botanic Gardens, Kew.
Manzanilla, V. & Bruneau, A. (2012). Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on duplicated copies of the sucrose synthase gene and plastid markers. Molec. Phylogenet. Evol. 65: 149 – 162.
McNeill, J., Barrie, F. R., Buck, W. R., Demoulin, V., Greuter, W., Hawksworth, D. L., Herendeen, P. S., Knapp, S., Marhold, K., Prado, J., Prud'homme van Reine, W. F., Smith, G. F., Wiersema, J. H. & Turland, N. J. (eds) (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Veg. 154. Koeltz Scientific Books.
Moreira, F. M. de S., Silva M. F. da & de Faria, S. M.(1992). Occurrence of nodulation in legume species in the Amazon region of Brazil. New Phytol. 121: 563 – 570.
Ribeiro, J. E. L. da S., Hopkins, M. J. G., Vicentini, A., Sothers, C. A., Costa, M. A. da S., Brito, J. M. de, Souza, M. A. D. de, Martins, L. H. P., Lohmann, L. G., Assunção, P. A. C. L., Pereira, E. da C., Silva, C. F. da, Mesquita, M. R. & Procópio, L. C. (1999). Flora da Reserva Ducke, Guia de identificação das plantas vasculares de uma floresta de terra-firme na Amazônia Central. INPA, Manaus.
Richter, H. G. & Dallwitz, M. J. (2009). Commercial timbers: descriptions, illustrations, identification, and information retrieval. In English, French, German, Portuguese, and Spanish. Version: 25th June 2009. http://delta-intkey.com
Sampaio, T. (1987). O tupi na geografia nacional, 5th ed. E. Nacional, São Paulo.
Sorsa, P. (1969). Pollen morphological studies on the Mimosaceae. Ann. Bot. Fenn. 6: 1 – 34.
Sprent, J. I. (2001). Nodulation in Legumes. Royal Botanic Gardens, Kew.
Thiers, B. [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Gardenʼs Virtual Herbarium. Published at: http://sweetgum.nybg.org/ih/
Acknowledgements
The authors thank the artist Margaret Tebbs for the elegant botanical line drawing, Domingos A. Folli for photographs in Fig. 2, and George Gosline for preparing the map. We also thank Nick Turland for advice with nomenclatural issues; Catia Canteiro for help with the conservation assessments; Ana Paula Fortuna for comments on the derivation of the common name of Dinizia jueirana-facao; Haroldo C. de Lima, Mike Hopkins and especially E. Simone Gurgel for assisting with the location of type material of D. excelsa; Pat Herendeen for providing pertinent fossil literature; Peter Gasson for data on wood; Sergio Faria and Janet Sprent for comment on nitrogen fixation; Aurelie Grall for translation from French of part of Ducke's protologue to D. excelsa; and Simon Mayo for information about J. P. Diniz. We also thank Bente Klitgård for helpful suggestions on an early draft of the paper, and Matt Lavin and one anonymous reviewer for their comments. The molecular phylogenetic analyses were supported by a grant from the Natural Sciences and Engineering Research Council of Canada to AB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lewis, G.P., Siqueira, G.S., Banks, H. et al. The majestic canopy-emergent genus Dinizia (Leguminosae: Caesalpinioideae), including a new species endemic to the Brazilian state of Espírito Santo. Kew Bull 72, 48 (2017). https://doi.org/10.1007/s12225-017-9720-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s12225-017-9720-7