Abstract
Francisella tularensis is the causative agent of the potentially lethal disease tularemia. Due to a low infectious dose and ease of airborne transmission, Francisella is classified as a category A biological agent. Despite the possible risk to public health, there is no safe and fully licensed vaccine. A potential vaccine candidate, an attenuated live vaccine strain, does not fulfil the criteria for general use. In this review, we will summarize existing and new candidates for live attenuated and subunit vaccines.
Similar content being viewed by others
Introduction—Francisella tularensis
Francisella tularensis (F. tularensis) is a non-motile, gram-negative, facultative intracellular pathogen that is the etiological agent of the potentially lethal disease tularemia in both humans and animals. This species is considered a biological weapon and classified as a category A bioterrorism agent by the US Centers for Disease Control and Prevention (Khan et al. 2000) due to its high infectivity, potential airborne transmission and ability to cause severe disease. During the Cold War, F. tularensis belonged to the group of agents produced and stockpiled by the former Soviet Union and the USA (reviewed in Dennis et al. 2001). In 1970, the World Health Organization committee categorized F. tularensis as a biological threat and estimated that the dispersal of 50 kg of its aerosolized virulent form over an urban area with five million inhabitants would result in 250,000 incapacitating causalities and 19,000 deaths (World Health Organization 1970). Today’s major concerns are the misuse of F. tularensis during possible terrorist attacks.
F. tularensis belongs to the class γ-Proteobacteria, family Francisellaceae and genus Francisella (Forsman et al. 1994; World Health Organization 2007). The species F. tularensis is divided into three subspecies: tularensis, holarctica and mediasiatica, which vary in their pathogenicity and geographic distribution (Oyston 2008). F. tularensis subsp. tularensis (classified as type A) is found predominantly in North America and consists of two different genetic sub-populations, AI and AII (Johansson et al. 2004), which are characterized by the extreme virulence, as less than 10 bacteria can lead to lethal disease (reviewed in Tärnvik and Berglund 2003). F. tularensis subsp. holarctica (type B) occurs primarily in the Northern Hemisphere and causes a milder form of tularemia. F. tularensis subsp. mediasiatica was detected in Central Asia, and its virulence resembles the holarctica subspecies. The species novicida, isolated in North America and Australia, is rarely responsible for human tularemia (reviewed in Pechous et al. 2009).
Natural hosts for F. tularensis include lagomorphs, rodents, carnivores, ungulates, marsupials, amphibians, birds, fish and invertebrates (Mörner 1992). However, despite the wide distribution of Francisella in numerous wildlife species, its primary reservoirs remain unknown. Natural infection can be transmitted to humans through arthropod vectors, such as ticks, flies or mosquitoes, or by direct contact during handling of infected animals, drinking of contaminated water or inhaling of aerosols (Mörner 1992; Tärnvik and Berglund 2003).
Tularemia
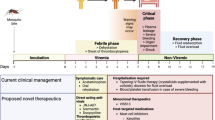
Tularemia is an acute febrile disease whose severity depends on the route of infection and virulence of the strain (Ellis et al. 2002). The incubation period normally ranges from 3 to 5 days; however, the period may be extended to up to 21 days. In the early phase of infection, tularemia is frequently misdiagnosed because disease symptoms resemble flu symptoms, such as high fever, body aches, and swollen lymph nodes. The most common form of tularemia is ulceroglandular tularemia, which is usually spread through vector-borne transmission (reviewed by Tärnvik and Berglund 2003). A painless ulcer develops at the site of inoculation followed by enlargement of regional lymph nodes (Ohara et al. 1991). The infrequent clinical form, oculoglandular tularemia, occurs after direct contact of the eye with the bacteria. Oropharyngeal tularemia, which is accompanied by stomatitis and pharyngitis, results from contaminated food or water intake (reviewed by Tärnvik and Berglund 2003; World Health Organization 2007). The most severe form is respiratory tularemia, which is caused by the inhalation of aerosolized F. tularensis subsp. tularensis. The mortality rate for respiratory tularemia ranges from 30 to 60 % without effective antibiotic therapy (reviewed by Tärnvik and Berglund 2003). Treatment successfully resolves infection when administered in the early phase of infection. Antibiotics of choice are aminoglycosides, tetracyclines, chloramphenicol and quinolones (reviewed by Dennis et al. 2001).
The human immune response to tularemia was described in naturally infected patients or live vaccine strain (LVS) vaccinated volunteers (Koskela and Herva 1982; Koskela and Salminen 1985; Surcel et al. 1991; Sjöstedt et al. 1992a; Poquet et al. 1998; Ericsson et al. 1994). Considering that F. tularensis is an intracellular pathogen, it was thought that a cell-mediated immune response is required to clear infection. CD4+ and CD8+ T cells are detectable 2 weeks post-infection, as well as proinflammatory cytokines, including interferon-γ (IFN-γ), TNF-α and interleukin 2 (IL-2) (Koskela and Herva 1982; Surcel et al. 1991; Sjöstedt et al. 1992a). The cell-mediated response is long-lasting and even inducible 30 years after the onset of disease (Ericsson et al. 2001). In naturally infected individuals, phosphoantigen-directed Vγ9/Vδ2 T cells arise during the first week after infection (Poquet et al. 1998). Human peripheral blood cells show increased expression of IFN-γ-regulated genes within 2–3 days post-infection (Andersson et al. 2006). In respect to humoral immunity, the production of specific IgM, IgA and IgG antibodies reaches its highest levels at 1–2 months and persists 0.5 to 11 years post-infection (Koskela and Salminen 1985). Recent studies in the murine model of tularemia showed that both components of adaptive immunity are critical for the induction of full protection against tularemia (Lavine et al. 2007; Cole et al. 2011; Kubelkova et al. 2012).
The current problem in tularemia prophylaxis is the lack of a vaccine. The only available prophylactic tool is LVS, which is not intended for public use due to its attenuation background. It is therefore vital to develop a new vaccine that will be safe and effective in inducing protective long-lasting immune response against respiratory challenge with the most virulent strain of F. tularensis. Currently, F. tularensis vaccine development has focused on developing live attenuated (Table 1) and subunit vaccines (Table 2).
Killed whole-cell vaccines
Killed whole-cell vaccines are composed of non-infectious modified bacterial suspensions. The earliest tularemia vaccine was developed using acetone extraction or phenolization by Foshay et al. (1942). The vaccine protected non-human primates against challenge with 740 CFU of F. tularensis subsp. tularensis Schu S4 (Schu S4); however, it caused symptoms of disease (Coriell et al. 1948) and was not efficient to protect against highly virulent strains in animal models (Foshay et al. 1942; Pechous et al. 2009).
Recent studies showed that protection induced by killed whole-cell vaccines are enhanced through the use of boosters and adjuvants. Eyles et al. (2008) found that intramuscular (i.m.)-delivered, inactivated LVS vaccination in conjunction with immune-stimulating complexes and immunostimulatory CpG oligonucleotides had a protective effect against aerosol challenge with F. tularensis subsp. holarctica, but not against low-dose aerosol challenge with Schu S4. Baron and co-workers (2007) determined that inactivated LVS administered via the intranasal (i.n.) route protected mice against i.n. infection with LVS, although only in combination with IL-12 administration.
Live attenuated vaccines
The first anti-Francisella live attenuated vaccine was generated from F. tularensis subsp. holarctica, which was isolated in the former Soviet Union (Tigertt 1962). A sample of the vaccine was provided to the US where multiple passages of the strain led to the preparation of LVS (Eigelsbach and Downs 1961). The results obtained from vaccine trials in humans showed that LVS induced protective immunity against a low-dose aerosol challenge with Schu S4 (McCrumb 1961). However, LVS has not been officially licensed by the Food and Drug Administration as a human vaccine due to an unknown mechanism of attenuation, the instability of colony phenotype and the partial virulence after vaccination via the aerosol route (Hornick and Eigelsbach 1966; Hartley et al. 2006; Petrosino et al. 2006).
Currently, live attenuated vaccines are prepared from live organisms and take into account the balance between attenuation and immunogenicity. Namely, over-attenuation could lead to the loss of partial bacterial virulence and an insufficient protective immune response. These types of vaccines are constructed by deleting genes involved in metabolic and virulence pathways, which are also necessary for F. tularensis intracellular replication and in vivo survival.
Mutations in genes involved in Francisella metabolic pathways
Screening for genes involved in purine biosynthetic pathways in the Schu S4 strain revealed novel candidates for live attenuated vaccines (Prior et al. 2001). F. novicida mutants ΔpurA, ΔpurCD and ΔpurM were attenuated in mice; protection against challenge with a homologous wild-type strain was not observed (Tempel et al. 2006; Quarry et al. 2007). Mutant ΔpurF protected mice against intraperitoneal (i.p.) challenge with F. novicida, but not against the virulent Schu S4 strain (Quarry et al. 2007). In another study, an attenuated LVS mutant lacking the purine biosynthetic locus ΔpurMCD protected against LVS lethal challenge (Pechous et al. 2006); however, a single dose of this vaccine did not demonstrate a protective effect against i.n. or intradermal (i.d.) Schu S4 infection. In contrast, i.n. immunization with the Schu S4 mutant ΔpurMCD protected against i.n. challenge with a parental strain; however, the challenge’s outcome was influenced by the side effects of immunization (Pechous et al. 2008). Targeted deletion of the genes guaA and guaB in LVS leads to the attenuation in mice and to the stimulation of a protective immune response to i.p. challenge with a lethal dose of the parental strain (Santiago et al. 2009). However, Schu S4 mutants were not able to protect against the wild-type strain (Santiago et al. 2015).
The capsule synthesis gene (capB) encodes an ATP-dependent ligase that is involved in capsule polysaccharide biosynthesis (Larsson et al. 2005). An LVS mutant with a targeted deletion in capB is significantly attenuated in mice, and its protective effect against i.n. challenge with a dose 10-fold greater than the LD50 of Schu S4 was 100 % (Jia et al. 2010). Jia and colleagues prepared a vaccine regimen from a highly attenuated LVS mutant, ΔcapB, which served as a primary immunogen, and rLm/iglC, which is an attenuated recombinant Listeria monocytogenes expressing F. tularensis protein IglC, which was used as a booster. Mice vaccinated with ΔcapB and rLm/iglC exhibited prolonged survival and mean time to death with a challenge dose 10 times the LD50 of aerosolized Schu S4 compared to immunization by ΔcapB or parental strain alone. The use of a booster also invoked increased T cell immunity and enhanced IFN-γ secretion (Jia et al. 2013).
Mutations in genes from the Francisella pathogenicity island
The Francisella pathogenicity island (FPI) is a ∼30 kb region of the Francisella genome (Nano et al. 2004) that is duplicated in all F. tularensis subspecies except F. novicida (Larsson et al. 2009). The FPI contains the IglABCD operon, as well as pdpABC, that is essential for virulence. The majority of FPI proteins forms the type VI-like secretion system and is required for phagosomal escape following intracellular replication (Nano et al. 2004; Straskova et al. 2012).
In LVS, deletion of the iglC gene, which encodes a 23 kDa intracellular growth locus protein, led to an intracellular macrophage growth defect and attenuation in mice (Golovliov et al. 2003). The same mutation in F. novicida provided protection against i.n. infection with the parental strain. These effects were mediated by induction of Th1-type cytokine and antibody response (Pammit et al. 2006). In contrary, ΔiglC of Schu S4 origin did not protect mice against aerosol exposure to type A F. tularensis (Twine et al. 2005). Cong and colleagues (2009) prepared the ΔiglB mutant of F. novicida U112, which protected mice against pulmonary challenge with the virulent Schu S4 strain. In a recent study, the F. novicida mutant ΔiglB, which expresses the D1 domain of FljB flagellin from Salmonella typhimurium (S. typhimurium) and is a potent Toll-like receptor 5 (TLR5) agonist, was constructed. Oral vaccination with the construct protected rats against pulmonary challenge with Schu S4 (Cunningham et al. 2014). In addition, the deletion of another FPI gene, iglH, in the FSC200 strain established an attenuated phenotype that protected mice against subcutaneous challenge with a fully virulent, homologous wild-type strain (Straskova et al. 2012); thus, these studies underline the potential of FPI genes in the development of live attenuated vaccines.
To survive, Francisella reacts to stimuli from its surroundings and, in response, regulates virulence factor production. Several factors were identified to regulate FPI gene expression and include the following: FevR, MglA, PmrA and SspA (Charity et al. 2007; Mohapatra et al. 2007). Deletion of either the mglA or pmrA genes in F. novicida led to the attenuation of virulence in mice (Lauriano et al. 2004; Mohapatra et al. 2007), although only the ΔpmrA mutant protected against challenge with the parental strain but not the Schu S4 strain (Mohapatra et al. 2007).
Mutations in various Francisella genes
Lipopolysaccharide (LPS), which consists of lipid A, core oligosaccharide and O-polysaccharide (O-PS) are the outer membrane components of a majority of gram-negative bacteria. In case of Francisella, these components are designed to support the pathogenic behaviour of bacteria (Okan and Kasper 2013). Due to the unusual tetraacylated structure of lipid A (Vinogradov et al. 2002), Francisella is able to evade detection by TLR4 (Dueñas et al. 2006). Attempts to mutate the LVS genes wbtA and wbtI, which are responsible for biosynthesis of O-PS, led to loss of O-PS and the attenuation of the strain’s virulence in mice. Moreover, mutants were able to protect against i.p. challenge with the parental strain (Raynaud et al. 2007; Li et al. 2007). Consistent with previous studies, the deletion of the gene wzy, which encodes O-PS polymerase, in LVS caused the strain to be highly attenuated in mice and demonstrated the protective effect of i.n. vaccination against i.n. challenge with the parental strain and virulent strain Schu S4 (Kim et al. 2012).
Acid phosphatases are the enzymes required for hydrolysis of phosphomonoesters, and they are the major virulence factors because of their connection to intracellular survival through repression of the oxidative burst in phagosomes (Reilly et al. 1996). Mohapatra and colleagues (2008) observed that the F. novicida quadrupole mutant, which lacks genes acpA, acpB, acpC and hapA, showed impaired phosphatase activity, phagosomal escape and intracellular survival in vitro and in mice, and its attenuated phenotype provided protection against F. novicida challenge.
Another bacterial protein involved in elimination of reactive oxygen intermediates is iron superoxide dismutase, which is encoded by the gene sodB. An LVS mutant, sodB, led to a significant attenuation of virulence in mice (Bakshi et al. 2006) and provided greater protection when compared to LVS administration after i.n. challenge with a lethal dose of Schu S4 (Bakshi et al. 2008).
The role of KatG is to catalyze bactericidal molecules, including H2O2 and ONOO−. Intracellular growth of LVS or the Schu S4 mutant ΔkatG was not affected, although mutants showed enhanced susceptibility to H2O2 during in vitro analysis. The results from the i.d. immunization study demonstrated attenuation of the LVS mutant ΔkatG compared to the homologous wild-type strain. However, no differences were detected between the Schu S4 mutant ΔkatG and corresponding wild-type strain (Lindgren et al. 2007).
The type IV pili (Tfp) are multifunctional, flexible adhesive fibres expressed in many gram-negative bacteria (Chakraborty et al. 2008). Genome analysis of Francisella revealed genes required for the expression of Tfp system (Larsson et al. 2005). The pilin PilA is considered a critical virulence factor for the type B strain in the mouse model, as its deletion results in attenuation and an inability of bacteria to spread from the original site of infection (Forslund et al. 2006). Consistent with this finding, the mutation of other Tfp components, such as PilF, PilT, PilE5 and PilE6 in the LVS strain, led to the virulence attenuation (Chakraborty et al. 2008; Ark and Mann 2011). Moreover, Forslund et al. (2010) observed that mice infected with in-frame deletion mutants of the genes pilA, pilC and pilQ in Schu S4 strain experienced a moderately delayed time to death.
In gram-negative bacteria, the formation of disulphide bonds in many proteins (including virulence factors) depends on the DsbA protein (Senitkova et al. 2011). A mutant lacking the gene FTT_1103, which encodes a dsbA homologue in Schu S4, was unable to escape phagosomes. The strain was attenuated in mice and showed a protective effect against i.n. Schu S4 challenge in BALB/c or C57BL/6 mice (Qin et al. 2009). Similar results were obtained for BALB/c mice infected with the ΔdsbA mutant on the FSC200 background (Straskova et al. 2015).
Intradermal immunization with the deletion mutant of chaperone ClpB in Schu S4 protected BALB/c mice against respiratory challenge with a homologous wild-type strain (Twine et al. 2012). Moreover, Golovliov et al. (2013) observed that a Schu S4 mutant exhibited an enhanced protective effect when compared to a mutant in F. tularensis subsp. holarctica FSC200 (FSC200).
During the Francisella intracellular life cycle, the expression of FTT_1676 and FTT_0369c genes is upregulated (Wehrly et al. 2009). The genes encode a glycosylated membrane lipoprotein (Balonova et al. 2012) and the Sel1-family tetratricopeptide repeat-containing protein, respectively. Inactivation of both genes led to attenuation in mice (Wehrly et al. 2009). Rockx-Brouwer et al. found that i.d. inoculation with a low concentration of both mutants were protective against i.n. or i.d. challenge with Schu S4. However, the degree of protection correlated with the replication ability of mutants in the host (Rockx-Brouwer et al. 2012). In a recent study, F. novicida lacking an orthologue of FTT_1676, transposon mutant FTN_0109, displayed impaired intracellular growth. Authors observed a complete protective effect against pulmonary infection with 10 LD50 of LVS in BALB/c mice, whereas intratracheal challenge with 25 LD50 of Schu S4 provided partial protection of Fisher 344 rats against the same dose of LVS (Cunningham et al. 2015).
Subunit vaccines
Subunit vaccines are considered a safe vaccine because of their composition, which consists of synthesized or isolated microbial antigens. In the case of Francisella, there are several bacterial structures that are considered potential subunit vaccines. One bacterial structure is Francisella LPS, which was able to induce some degree of protective immune response. Intradermal treatment of mice with LPS isolated from LVS provided protection against lethal challenge with a homologous strain (Dreisbach et al. 2000), but not against Schu S4 (Fulop et al. 2001). LPS purified from Schu S4 was able to extend the time to death in mice, but it was not able to protect against challenge with the parental strain (Prior et al. 2003). The failure of LPS to evoke a fully protective immune response probably results from its inability to stimulate robust cell-mediated immunity. In theory, the poor protection ability of LPS may be improved by adjuvant systems that induce T cell immunity. Richard et al. explored the immunogenic properties of synthetic nanoparticles prepared from catanionic surfactant vesicles that were activated by the incorporation of Francisella components. Adjuvant-associated LPS from LVS was used as a vaccine. Treated mice were protected against i.p. challenge with LVS yet remained vulnerable to i.n. infection with Schu S4. Authors enhanced effectiveness by incorporating components from LVS or Schu S4 whole bacterial lysates. However, they reached only partial protection against i.n. challenge with Schu S4 (Richard et al. 2014).
The weak proinflammatory nature of LPS turned our attention to Francisella immunogenic proteins. The partial protective effect against lethal respiratory challenge with LVS in mice was induced by Francisella heat shock protein DnaK and surface lipoprotein Tul4 with co-administration of GPI-0100 i.n. as an adjuvant (Ashtekar et al. 2012). Recently, Banik et al. prepared a multivalent subunit vaccine by using tobacco mosaic virus as delivery system in combination with Francisella proteins, Tul4, DnaK and OmpA. Treated mice were protected against lethal LVS infection (Banik et al. 2015). Tul4 served as a basis for a subunit vaccine constructed from a replication-incompetent adenovirus carrying a codon-optimized gene for its expression, Ad-opt/Tul4. As a result, 60 % of mice were protected against i.p. infection with LVS following an i.m. immunization with a construct and two boosters (Kaur et al. 2012). In another report, authors vaccinated using a construct from an attenuated Δasd Δcya Δcrp S. typhimurium mutant carrying Tul4. The construct provided partial protection against intravenous challenge with LVS (Sjöstedt et al. 1992b). Similar results were obtained by Golovliov et al. (1995) who tested Tul4 in combination with immunostimulating complexes.
Jia et al. used an approach which employed attenuated rLm as a delivery vehicle that stably expressed various Francisella proteins, including AcpA, Bfr, DnaK, GroEL, KatG, Pld or IglC. However, only i.d. immunization with IglC-producing rLm evoked sufficient protection against i.n. lethal challenge with LVS or aerosolized Schu S4 (Jia et al. 2009).
Another candidate for a potential subunit vaccine, outer membrane protein A (FopA), was utilized as a recombinant protein incorporated into liposomes. Immunized mice showed a specific antibody response, and they were protected against a lethal i.n. and i.d. challenge with LVS, but not with the type A strain. Passively transferred FopA-immune serum to the naive mice protected against LVS infection (Hickey et al. 2011).
Conclusions
A tularemia vaccine has to fulfil various criteria; it must be safe and should be able to induce complete long-lasting protective immunity in individuals of all ages and with diverse levels of immunocompetence. The vaccine should protect against respiratory tularemia invoked not only by the most virulent type A strain Schu S4 but also by other less virulent strains. Despite intensive research in this area, there are still serious hurdles that impede the significant progress in tularemia vaccine development. Currently, LVS strain represent the most extensively studied vaccine candidate; however, as it was already mentioned, it does not provide sufficient protection against respiratory infection with Francisella type A strains and the molecular basis of its attenuation has not been clarified, as well. On the other side, a plethora of new promising candidates for live attenuated vaccines with defined gene deletion and good protective efficacy against type A strains have been prepared. Nevertheless, their experimental and clinical testing is in its infancy. Additionally, they usually exhibit high variability in their protective effects that is associated with the selection of the vaccination strain, dose and route of administration. The selection of proper animal model represents another weak point in a vaccine development. Mice are generally used for vaccination studies, but they are more sensitive to primary pulmonary infection with Francisella tularensis than humans; therefore, their usage for evaluation of basic mechanisms of Francisella pathogenesis and immune response to this microbe is not sufficient. It is necessary to combine several animal models in order to confirm the potential benefit of experimental vaccine for humans. Last but not the least, the mechanism of vaccine-elicited immune response has not been elucidated in a sufficient way up to now. This knowledge is a prerequisite for the identification of reliable correlates of post-vaccination protection.
Although live attenuated vaccines show promising protective effects, current trends in prophylaxis development, due to safety reasons, favour subunit vaccines rather than the live attenuated strains. Because Francisella is an intracellular pathogen, a Francisella subunit vaccine needs to induce cell-based response. However, the identification of T cell specific epitopes is not trivial. One of the most promising approaches is whole genome immunoinformatic analysis, which detects immunogenic Francisella peptides that bind to MHCI (Rotem et al. 2014; Zvi et al. 2011). Alternatively, a protein array-based approach can identify epitopes from MHCII complexes from various serological targets (Valentino et al. 2011). It is expected that these new “omics” approaches can provide novel peptides epitopes for the development of the effective subunit vaccines.
References
Andersson H, Hartmanova B, Bäck E et al (2006) Transcriptional profiling of the peripheral blood response during tularemia. Genes Immun 7:503–513. doi:10.1038/sj.gene.6364321
Ark NM, Mann BJ (2011) Impact of Francisella tularensis pilin homologs on pilus formation and virulence. Microbial Pathogenesis 51:110–120. doi:10.1016/j.micpath.2011.05.001
Ashtekar AR, Katz J, Xu Q, Michalek SM (2012) A mucosal subunit vaccine protects against lethal respiratory infection with Francisella tularensis LVS. PLoS ONE 7, e50460. doi:10.1371/journal.pone.0050460
Bakshi CS, Malik M, Regan K et al (2006) Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol 188:6443–6448. doi:10.1128/JB.00266-06
Bakshi CS, Malik M, Mahawar M et al (2008) An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine 26:5276–5288. doi:10.1016/j.vaccine.2008.07.051
Balonova L, Mann BF, Cerveny L, et al (2012) Characterization of protein glycosylation in Francisella tularensis subsp. holarctica. Mol Cell Proteomics. doi: 10.1074/mcp.M111.015016
Banik S, Mansour AA, Suresh RV et al (2015) Development of a multivalent subunit vaccine against tularemia using tobacco mosaic virus (TMV) based delivery system. PLoS One. doi:10.1371/journal.pone.0130858
Baron SD, Singh R, Metzger DW (2007) Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun 75:2152–2162. doi:10.1128/IAI.01606-06
Chakraborty S, Monfett M, Maier TM et al (2008) Type IV pili in Francisella tularensis: roles of pilF and pilT in fiber assembly, host cell adherence, and virulence. Infect Immun 76:2852–2861. doi:10.1128/IAI.01726-07
Charity JC, Costante-Hamm MM, Balon EL et al (2007) Twin RNA polymerase-associated proteins control virulence gene expression in Francisella tularensis. PLoS Pathog. doi:10.1371/journal.ppat.0030084
Cole LE, Mann BJ, Shirey KA et al (2011) Role of TLR signaling in Francisella tularensis-LPS-induced, antibody-mediated protection against Francisella tularensis challenge. J Leukoc Biol 90:787–797. doi:10.1189/jlb.0111014
Cong Y, Yu J-J, Guentzel MN et al (2009) Vaccination with a defined Francisella tularensis subsp. novicida pathogenicity island mutant (ΔiglB) induces protective immunity against homotypic and heterotypic challenge. Vaccine 27:5554–5561. doi:10.1016/j.vaccine.2009.07.034
Coriell LL, King EO, Smith MG (1948) Studies on tularemia; observations on tularemia in normal and vaccinated monkeys. J Immunol 58:183–202
Cunningham AL, Dang KM, Yu J-J et al (2014) Enhancement of vaccine efficacy by expression of a TLR5 ligand in the defined live attenuated Francisella tularensis subsp. novicida strain U112ΔiglB::fljB. Vaccine 32:5234–5240. doi:10.1016/j.vaccine.2014.07.038
Cunningham AL, Guentzel MN, Yu J-J et al (2015) Vaccination with the live attenuated Francisella novicida mutant FTN0109 protects against pulmonary tularemia. World J Vaccines 05:25–36. doi:10.4236/wjv.2015.51004
Dennis DT, Inglesby TV, Henderson DA et al (2001) Tularemia as a biological weapon: medical and public health management. JAMA 285:2763–2773
Dreisbach VC, Cowley S, Elkins KL (2000) Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun 68:1988–1996
Dueñas AI, Aceves M, Orduña A et al (2006) Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol 18:785–795. doi:10.1093/intimm/dxl015
Eigelsbach HT, Downs CM (1961) Prophylactic effectiveness of live and killed tularemia vaccines I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol 87:415–425
Ellis J, Oyston PCF, Green M, Titball RW (2002) Tularemia. Clin Microbiol Rev 15:631–646. doi:10.1128/CMR.15.4.631-646.2002
Ericsson M, Sandström G, Sjöstedt A, Tärnvik A (1994) Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis 170:110–114
Ericsson M, Kroca M, Johansson T et al (2001) Long-lasting recall response of CD4+ and CD8+ alphabeta T cells, but not gammadelta T cells, to heat shock proteins of Francisella tularensis. Scand J Infect Dis 33:145–152
Eyles JE, Hartley MG, Laws TR et al (2008) Protection afforded against aerosol challenge by systemic immunisation with inactivated Francisella tularensis live vaccine strain (LVS). Microbial Pathogenesis 44:164–168. doi:10.1016/j.micpath.2007.08.009
Forslund A-L, Kuoppa K, Svensson K et al (2006) Direct repeat-mediated deletion of a type IV pilin gene results in major virulence attenuation of Francisella tularensis. Mol Microbiol 59:1818–1830. doi:10.1111/j.1365-2958.2006.05061.x
Forslund A-L, Salomonsson EN, Golovliov I et al (2010) The type IV pilin, PilA, is required for full virulence of Francisella tularensis subspecies tularensis. BMC Microbiology 10:227. doi:10.1186/1471-2180-10-227
Forsman M, Sandström G, Sjöstedt A (1994) Analysis of 16S ribosomal DNA sequences of Francisella strains and utilization for determination of the phylogeny of the genus and for identification of strains by PCR. Int J Syst Bacteriol 44:38–46. doi:10.1099/00207713-44-1-38
Foshay L, Hesselbrock WH, Wittenberg HJ, Rodenberg AH (1942) Vaccine prophylaxis against tularemia in man. Am J Public Health Nations Health 32:1131–1145
Fulop M, Mastroeni P, Green M, Titball RW (2001) Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine 19:4465–4472. doi:10.1016/S0264-410X(01)00189-X
Golovliov I, Ericsson M, Åkerblom L et al (1995) Adjuvanticity of ISCOMs incorporating a T cell-reactive lipoprotein of the facultative intracellular pathogen Francisella tularensis. Vaccine 13:261–267. doi:10.1016/0264-410X(95)93311-V
Golovliov I, Sjöstedt A, Mokrievich A, Pavlov V (2003) A method for allelic replacement in Francisella tularensis. FEMS Microbiology Letters 222:273–280. doi:10.1016/S0378-1097(03)00313-6
Golovliov I, Twine SM, Shen H et al (2013) A ΔclpB mutant of Francisella tularensis subspecies holarctica strain, FSC200, is a more effective live vaccine than F. tularensis LVS in a mouse respiratory challenge model of tularemia. PLoS One doi. doi:10.1371/journal.pone.0078671
Hartley G, Taylor R, Prior J et al (2006) Grey variants of the live vaccine strain of Francisella tularensis lack lipopolysaccharide O-antigen, show reduced ability to survive in macrophages and do not induce protective immunity in mice. Vaccine 24:989–996. doi:10.1016/j.vaccine.2005.08.075
Hickey AJ, Hazlett KRO, Kirimanjeswara GS, Metzger DW (2011) Identification of Francisella tularensis outer membrane protein A (FopA) as a protective antigen for tularemia. Vaccine 29:6941–6947. doi:10.1016/j.vaccine.2011.07.075
Hornick RB, Eigelsbach HT (1966) Aerogenic immunization of man with live tularemia vaccine. Bacteriol Rev 30:532–538
Jia Q, Lee B-Y, Clemens DL et al (2009) Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized type A F. tularensis. Vaccine 27:1216–1229. doi:10.1016/j.vaccine.2008.12.014
Jia Q, Lee B-Y, Bowen R et al (2010) A Francisella tularensis live vaccine strain (LVS) mutant with a deletion in capB, encoding a putative capsular biosynthesis protein, is significantly more attenuated than LVS yet induces potent protective immunity in mice against F. tularensis challenge. Infect Immun 78:4341–4355. doi:10.1128/IAI.00192-10
Jia Q, Bowen R, Sahakian J et al (2013) A heterologous prime-boost vaccination strategy comprising the Francisella tularensis live vaccine strain capB mutant and recombinant attenuated Listeria monocytogenes expressing F. tularensis IglC induces potent protective immunity in mice against virulent F. tularensis aerosol challenge. Infect Immun 81:1550–1561. doi:10.1128/IAI.01013-12
Johansson A, Farlow J, Larsson P et al (2004) Worldwide genetic relationships among Francisella tularensis isolates determined by multiple-locus variable-number tandem repeat analysis. J Bacteriol 186:5808–5818. doi:10.1128/JB.186.17.5808-5818.2004
Kaur R, Chen S, Arévalo MT et al (2012) Protective immunity against tularemia provided by an adenovirus-vectored vaccine expressing Tul4 of Francisella tularensis. Clin Vaccine Immunol 19:359–364. doi:10.1128/CVI.05384-11
Khan AS, Levitt AM, Sage MJ et al (2000) Biological and chemical terrorism: strategic plan for preparedness and response: recommendations of the CDC Strategic Planning Workgroup. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4904a1.htm. Accessed 20 January 2015
Kim T-H, Pinkham JT, Heninger SJ et al (2012) Genetic modification of the O-polysaccharide of Francisella tularensis results in an avirulent live attenuated vaccine. J Infect Dis 205:1056–1065. doi:10.1093/infdis/jir620
Koskela P, Herva E (1982) Cell-mediated and humoral immunity induced by a live Francisella tularensis vaccine. Infect Immun 36:983–989
Koskela P, Salminen A (1985) Humoral immunity against Francisella tularensis after natural infection. J Clin Microbiol 22:973–979
Kubelkova K, Krocova Z, Balonova L et al (2012) Specific antibodies protect gamma-irradiated mice against Francisella tularensis infection. Microbial Pathogenesis 53:259–268. doi:10.1016/j.micpath.2012.07.006
Larsson P, Oyston PCF, Chain P et al (2005) The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet 37:153–159. doi:10.1038/ng1499
Larsson P, Elfsmark D, Svensson K et al (2009) Molecular evolutionary consequences of niche restriction in Francisella tularensis, a facultative intracellular pathogen. PLoS Pathog. doi:10.1371/journal.ppat.1000472
Lauriano CM, Barker JR, Yoon S-S et al (2004) MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. PNAS 101:4246–4249. doi:10.1073/pnas.0307690101
Lavine CL, Clinton SR, Angelova-Fischer I et al (2007) Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol 37:3007–3020. doi:10.1002/eji.200737620
Li J, Ryder C, Mandal M et al (2007) Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology 153:3141–3153. doi:10.1099/mic.0.2007/006460-0
Lindgren H, Shen H, Zingmark C et al (2007) Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect Immun 75:1303–1309. doi:10.1128/IAI.01717-06
McCrumb FR (1961) Aerosol infection of man with Pasteurella tularensis. Bacteriol Rev 25:262–267
Mohapatra NP, Soni S, Bell BL et al (2007) Identification of an orphan response regulator required for the virulence of Francisella spp. and transcription of pathogenicity island genes. Infect Immun 75:3305–3314. doi:10.1128/IAI.00351-07
Mohapatra NP, Soni S, Reilly TJ et al (2008) Combined deletion of four Francisella novicida acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect Immun 76:3690–3699. doi:10.1128/IAI.00262-08
Mörner T (1992) The ecology of tularaemia. Rev - Off Int Epizoot 11:1123–1130
Nano FE, Zhang N, Cowley SC et al (2004) A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol 186:6430–6436. doi:10.1128/JB.186.19.6430-6436.2004
Ohara Y, Sato T, Fujita H et al (1991) Clinical manifestations of tularemia in Japan—analysis of 1,355 cases observed between 1924 and 1987. Infection 19:14–17
Okan NA, Kasper DL (2013) The atypical lipopolysaccharide of Francisella. Carbohydr Res 378:79–83. doi:10.1016/j.carres.2013.06.015
Oyston PCF (2008) Francisella tularensis: unravelling the secrets of an intracellular pathogen. J Med Microbiol 57:921–930. doi:10.1099/jmm.0.2008/000653-0
Pammit MA, Raulie EK, Lauriano CM et al (2006) Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun 74:2063–2071. doi:10.1128/IAI.74.4.2063-2071.2006
Pechous RD, Celli J, Penoske R et al (2006) Construction and characterization of an attenuated purine auxotroph in a Francisella tularensis live vaccine strain. Infect Immun 74:4452–4461. doi:10.1128/IAI.00666-06
Pechous RD, McCarthy TR, Mohapatra NP et al (2008) A Francisella tularensis Schu S4 purine auxotroph is highly attenuated in mice but offers limited protection against homologous intranasal challenge. PLoS ONE. doi:10.1371/journal.pone.0002487
Pechous RD, McCarthy TR, Zahrt TC (2009) Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev 73:684–711. doi:10.1128/MMBR.00028-09
Petrosino JF, Xiang Q, Karpathy SE et al (2006) Chromosome rearrangement and diversification of Francisella tularensis revealed by the type B (OSU18) genome sequence. J Bacteriol 188:6977–6985. doi:10.1128/JB.00506-06
Poquet Y, Kroca M, Halary F et al (1998) Expansion of Vgamma9 Vdelta2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun 66:2107–2114
Prior RG, Klasson L, Larsson P et al (2001) Preliminary analysis and annotation of the partial genome sequence of Francisella tularensis strain Schu 4. J Applied Microbiology 91:614–620. doi:10.1046/j.1365-2672.2001.01499.x
Prior JL, Prior RG, Hitchen PG et al (2003) Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J Med Microbiol 52:845–851. doi:10.1099/jmm.0.05184-0
Qin A, Scott DW, Thompson JA, Mann BJ (2009) Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect Immun 77:152–161. doi:10.1128/IAI.01113-08
Quarry JE, Isherwood KE, Michell SL et al (2007) A Francisella tularensis subspecies novicida purF mutant, but not a purA mutant, induces protective immunity to tularemia in mice. Vaccine 25:2011–2018. doi:10.1016/j.vaccine.2006.11.054
Raynaud C, Meibom KL, Lety M-A et al (2007) Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect Immun 75:536–541. doi:10.1128/IAI.01429-06
Reilly TJ, Baron GS, Nano FE, Kuhlenschmidt MS (1996) Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem 271:10973–10983. doi:10.1074/jbc.271.18.10973
Richard K, Mann BJ, Stocker L et al (2014) Novel catanionic surfactant vesicle vaccines protect against Francisella tularensis LVS and confer significant partial protection against F. tularensis Schu S4 strain. Clin Vaccine Immunol 21:212–226. doi:10.1128/CVI.00738-13
Rockx-Brouwer D, Chong A, Wehrly TD et al (2012) Low dose vaccination with attenuated Francisella tularensis strain SchuS4 mutants protects against tularemia independent of the route of vaccination. PLoS ONE 7, e37752. doi:10.1371/journal.pone.0037752
Rotem S, Cohen O, Bar-Haim E, et al (2014) Protective immunity against lethal F. tularensis holarctica LVS provided by vaccination with selected novel CD8+ T cell epitopes. PLoS ONE 9:e85215. doi: 10.1371/journal.pone.0085215
Santiago AE, Cole LE, Franco A et al (2009) Characterization of rationally attenuated Francisella tularensis vaccine strains that harbor deletions in the guaA and guaB genes. Vaccine 27:2426–2436. doi:10.1016/j.vaccine.2009.02.073
Santiago AE, Mann BJ, Qin A, et al (2015) Characterization of Francisella tularensis Schu S4 defined mutants as live-attenuated vaccine candidates. Pathog Dis 73:ftv036. doi: 10.1093/femspd/ftv036
Senitkova I, Spidlova P, Hernychova L, Stulik J (2011) The disulfide bond formation and its relationship to bacterial pathogenicity of three important gram-negative bacteria. Mil Med Sci Lett 80:118–128
Sjöstedt A, Eriksson M, Sandström G, Tärnvik A (1992a) Various membrane proteins of Francisella tularensis induce interferon-gamma production in both CD4+ and CD8+ T cells of primed humans. Immunology 76:584–592
Sjöstedt A, Sandström G, Tärnvik A (1992b) Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect Immun 60:2855–2862
Straskova A, Cerveny L, Spidlova P et al (2012) Deletion of IglH in virulent Francisella tularensis subsp. holarctica FSC200 strain results in attenuation and provides protection against the challenge with the parental strain. Microbes and Infection 14:177–187. doi:10.1016/j.micinf.2011.08.017
Straskova A, Spidlova P, Mou S et al (2015) Francisella tularensis type B ΔdsbA mutant protects against type A strain and induces strong inflammatory cytokine and Th1-like antibody response in vivo. Pathog Dis. doi:10.1093/femspd/ftv058
Surcel HM, Syrjälä H, Karttunen R et al (1991) Development of Francisella tularensis antigen responses measured as T-lymphocyte proliferation and cytokine production (tumor necrosis factor alpha, gamma interferon, and interleukin-2 and -4) during human tularemia. Infect Immun 59:1948–1953
Tärnvik A, Berglund L (2003) Tularaemia. Eur Respir J 21:361–373. doi:10.1183/09031936.03.00088903
Tempel R, Lai X-H, Crosa L et al (2006) Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect Immun 74:5095–5105. doi:10.1128/IAI.00598-06
Tigertt WD (1962) Soviet viable Pasteurella tularensis vaccines a review of selected articles. Bacteriol Rev 26:354–373
Twine S, Byström M, Chen W et al (2005) A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun 73:8345–8352. doi:10.1128/IAI.73.12.8345-8352.2005
Twine S, Shen H, Harris G et al (2012) BALB/c mice, but not C57BL/6 mice immunized with a ΔclpB mutant of Francisella tularensis subspecies tularensis are protected against respiratory challenge with wild-type bacteria: association of protection with post-vaccination and post-challenge immune responses. Vaccine 30:3634–3645. doi:10.1016/j.vaccine.2012.03.036
Valentino MD, Maben ZJ, Hensley LL et al (2011) Identification of T-cell epitopes in Francisella tularensis using an ordered protein array of serological targets. Immunology 132:348–60. doi:10.1111/j.1365-2567.2010.03387.x
Vinogradov E, Perry MB, Conlan JW (2002) Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem 269:6112–6118
Wehrly TD, Chong A, Virtaneva K et al (2009) Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol 11:1128–1150. doi:10.1111/j.1462-5822.2009.01316.x
West TE, Pelletier MR, Majure MC et al (2008) Inhalation of Francisella novicida ΔmglA causes replicative infection that elicits innate and adaptive responses but is not protective against invasive pneumonic tularemia. Microbes and Infection 10:773–780. doi:10.1016/j.micinf.2008.04.008
World Health Organization (1970) Health aspects of chemical and biological weapons. http://apps.who.int/iris/bitstream/10665/39444/1/24039.pdf. Accessed 20 January 2015
World Health Organization (2007) WHO guidelines on Tularaemia. http://www.cdc.gov/tularemia/resources/whotularemiamanual.pdf. Accessed 20 January 2015
Zvi A, Rotem S, Bar-Haim E, et al (2011) Whole-genome immunoinformatic analysis of F. tularensis: predicted CTL epitopes clustered in hotspots are prone to elicit a T-cell response. PLoS ONE 6:e20050. doi: 10.1371/journal.pone.0020050
Acknowledgments
This work was supported by the Specific Research grant of Ministry of Education, Youth and Sports of Czech Republic (SV/FVZ201202) and by a long-term organization development plan 1011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Putzova, D., Senitkova, I. & Stulik, J. Tularemia vaccines. Folia Microbiol 61, 495–504 (2016). https://doi.org/10.1007/s12223-016-0461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-016-0461-z