Abstract
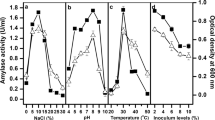
A halophilic isolate Thalassobacillus sp. LY18 producing extracellular amylase was isolated from the saline soil of Yuncheng Salt Lake, China. Production of the enzyme was synchronized with bacterial growth and reached a maximum level during the early stationary phase. The amylase was purified to homogeneity with a molecular mass of 31 kDa. Major products of soluble starch hydrolysis were maltose and maltotriose, indicating an α-amylase activity. Optimal enzyme activity was found to be at 70°C, pH 9.0, and 10 % NaCl. The α-amylase was highly stable over broad temperature (30–90°C), pH (6.0–12.0), and NaCl concentration (0–20 %) ranges, showing excellent thermostable, alkalistable, and halotolerant nature. The enzyme was stimulated by Ca2+, but greatly inhibited by EDTA, indicating it was a metalloenzyme. Complete inhibition by diethyl pyrocarbonate and β-mercaptoethanol revealed that histidine residue and disulfide bond were essential for enzyme catalysis. The surfactants tested had no significant effects on the amylase activity. Furthermore, it showed high activity and stability in the presence of water-insoluble organic solvents with log P ow ≥ 2.13.




Similar content being viewed by others
Abbreviations
- EDTA:
-
Ethylenediamine tetraacetic acid
- PAO:
-
Phenylarsine oxide
- DEPC:
-
Diethyl pyrocarbonate
- PMSF:
-
Phenylmethylsulfonyl fluoride
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Antranikian G, Vorgias CE, Bertoldo C (2005) Extreme environments as a resource for microorganisms and novel biocatalysts. Adv Biochem Eng Biotechnol 96:219–262
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cadenas Q, Engel PC (1994) Activity staining of halophilic enzymes: substitution of salt with zwitterions in non-denaturing electrophoresis. Biochem Mol Biol Int 33:785–792
Chakraborty S, Khopade A, Biao R, Jian W, Liu XY, Mahadik K, Chopade B, Zhang LX, Kokare C (2011) Characterization and stability studies on surfactant, detergent and oxidant stable α-amylase from marine haloalkaliphilic Saccharopolyspora sp. A9. J Mol Catal B Enzyme 68(1):52–58
Coronado MJ, Vargas C, Hofemeister J, Ventosa A, Nieto JJ (2000) Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Demirkan ES, Mikami B, Adachi M, Higas T, Utsumi S (2005) α-Amylase from B. amyloliquefaciens: purification, characterization, raw starch degradation and expression in E. coli. Process Biochem 40:2629–2636
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Finore I, Kasavi C, Aa P, Romano I, Oner ET, Kirdar B, Dipasquale L, Nicolaus B, Lama L (2011) Purification, biochemical characterization and gene sequencing of a thermostable raw starch digesting α-amylase from Geobacillus thermoleovorans subsp. stromboliensis subsp. nov. World J Microbiol Biotechnol 27:2425–2433
Fukushima T, Mizuki T, Echigo A, Inoue A, Usami R (2005) Organic solvent tolerance of halophilic α-amylase from a Haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9:85–89
García MT, Gallego V, Ventosa A, Mellado E (2005) Thalassobacillus devorans gen. nov., sp. nov., a moderately halophilic, phenol-degrading, Gram-positive bacterium. Int J Syst Evol Microbiol 55(Pt 5):1789–1795
Karbalaei-Heidari HR, Ziaee AA, Amoozegar MA (2007) Purification and biochemical characterization of a protease secreted by the Salinivibrio sp. strain AF-2004 and its behavior in organic solvents. Extremophiles 11:237–243
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li X, Yu HY (2011) Extracellular production of beta-amylase by a halophilic isolate, Halobacillus sp. LY9. J Ind Microbiol Biotechnol 38(11):1837–1843
Li X, Yu HY, Liu XX, Sun X (2011) Production and characterization of a novel extracellular metalloproteinase by a newly isolated moderate halophile, Halobacillus sp. LY6. Folia Microbiol (Praha) 56(4):329–334
Mamo G, Gessesse A (1999) Effect of cultivation conditions on growth and α-amylase production by a thermophilic Bacillus sp. Lett Appl Microbiol 29:61–65
Marhuenda-Egea FC, Bonete MJ (2002) Extreme halophilic enzymes in organic solvents. Curr Opin Biotechnol 13:385–389
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Prakash B, Vidyasagar M, Madhukumar MS, Muralikrishna G, Sreeramulu K (2009) Production, purification, and characterization of two extremely halotolerant, thermostable, and alkalistable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem 44:210–215
Ruiz DM, De Castro RE (2007) Effect of organic solvents on the activity and stability of an extracellular protease secreted by the haloalkaliphilic archaeon Natrialba magadii. J Ind Microbiol Biotechnol 34(2):111–115
Shafiei M, Ziaee AA, Amoozegar MA (2011) Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. strain F. J Ind Microbiol Biotechnol 38(2):275–281
Shafiei M, Ziaeea A-A, Amoozegar MA (2010) Purification and biochemical characterization of a novel SDS and surfactant stable, raw starch digesting, and halophilic α-amylase from a moderately halophilic bacterium Nesterenkonia sp. strain F. Process Biochem 45(5):694–699
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) α-Amylases from microbial sources-an overview on recent developments. Food Technol Biotechnol 44:173–184
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Zaks A, Klibanov AM (1988) Enzymatic catalysis in nonaqueous solvents. J Biol Chem 263(7):3194–3201
Acknowledgments
This work was financially supported by Shanxi Provincial Science and Technology Foundation (grants no. 20110021) and Natural Science Fund of Shanxi Province (grants no. 2011021031-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Yu, HY. Characterization of an organic solvent-tolerant α-amylase from a halophilic isolate, Thalassobacillus sp. LY18. Folia Microbiol 57, 447–453 (2012). https://doi.org/10.1007/s12223-012-0160-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0160-3