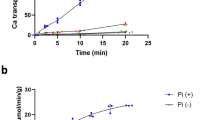
Abstract
The transport activity and substrate specificity of two chimeras consisting of S. cerevisiae Nha1p’s N-terminal regions (either first 125 or 184 AA) and the rest of the C. glabrata Cnh1p (up to the total protein length of 946 AA) were compared with those of the two native antiporters. Both chimeric transporters were functional upon expression in S. cerevisiae cells, their presence improved the ability of cells to grow in the presence of high external concentration of K+, Na+ or Rb+ (as chlorides), but not in the presence of the smallest cation (Li+). Cation efflux confirmed the ability of chimeras to export cations and showed their significantly reduced transport capacity compared to the wild-type proteins. Despite the very high level of primary sequence identity (87 %) between the S. cerevisiae and C. glabrata plasma-membrane Na+/H+ antiporters, various parts of these proteins are not exchangeable without affecting the antiporter’s transport capacity.
Similar content being viewed by others
Abbreviations
- AA:
-
amino-acid residues
- NT:
-
nucleotides
- TMS:
-
transmembrane segments
References
Arino J., Ramos J., Sychrova H.: Alkali-metal-cation transport and homeostasis in yeasts. Microbiol.Mol.Biol.Rev.75, 95–120 (2010).
Banuelos M.A., Sychrova H., Bleykasten-grosshans C., Souciet J.L., Potier S.: The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology144, 2749–2758 (1998).
Brett C.L., Donowitz M., Rao R.: Evolutionary origins of eukaryotic sodium/proton exchangers. Am.J.Physiol.Cell Physiol.288, C223–C239 (2005).
Hunte C., Screpanti E., Venturi M., Rimon A., Padan E., Michel H.: Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature435, 1197–1202 (2005).
Kamauchi S., Mitsui K., Ujike S., Haga M., Nakamura N., Inoue H., Sakajo S., Ueda M., Tanaka A., Kanazawa H.: Structurally and functionally conserved domains in the diverse hydrophilic carboxy-terminal halves of various yeast and fungal Na+/H+ antiporters (Nhalp). J.Biochem.(Tokyo)131, 821–831 (2002).
Kinclova O., Ramos J., Potier S., Sychrova H.: Functional study of the Saccharomyces cerevisiae Nha1p C-terminus. Mol.Microbiol.40, 656–668 (2001).
Kinclova-zimmermannova O., Zavrel M., Sychrova H.: Identification of conserved prolyl residue important for transport activity and the substrate specificity range of yeast plasma membrane Na+/H+ antiporters. J.Biol.Chem.280, 30638–30647 (2005).
Kinclova-zimmermannova O., Zavrel M., Sychrova H.: Importance of the seryl and threonyl residues of the fifth transmembrane domain to the substrate specificity of yeast plasma membrane Na+/H+ antiporters. Mol.Membr.Biol.23, 349–361 (2006).
Kinclova-zimmermannova O., Sychrova H.: Plasma-membrane Cnhl Na+/H+ antiporter regulates potassium homeostasis in Candida albicans. Microbiology153, 2603–2612 (2007).
Krauke Y., Sychrova H.: Functional comparison of plasma-membrane Na+/H+ antiporters from two pathogenic Candida species. BMC Microbiol.8, 80 doi: 10.1186/1471-2180-8-80 (2008).
Krauke Y., Sychrova H.: Cnh1 Na+/H+ antiporter and Ena1 Na+-ATPase play different roles in cation homeostasis and cell physiology of Candida glabrata. Fungal Gen.Biol., in press (2010).
Pribylova L., Papouskova K, Zavrel M., Souciet J.L., Sychrova H.: Exploration of yeast alkali metal cation/H+ antiporters: sequence and structure comparison. Folia Microbiol.51, 413–424 (2006).
Pribylova L., Papouskova K., Sychrova H.: The salt tolerant yeast Zygosaccharomyces rouxii possesses two plasma-membrane Na+/H+-antiporters (ZrNha1p and ZrSod2-22p) playing different roles in cation homeostasis and cell physiology. Fungal Gen.Biol.45, 1439–1447 (2008).
Saini P., Gaur N.A., Prasad R.: Chimeras of the ABC drug transporter Cdr1p reveal functional indispensability of transmembrane domains and nucleotide-binding domains, but transmembrane segment 12 is replaceable with the corresponding homologous region of the non-drug transporter Cdr3p. Microbiology152, 1559–1573 (2006).
Williams K.A.: Three-dimensional structure of the ion-coupled transport protein NhaA. Nature403, 112–115 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krauke, Y., Sychrová, H. Chimeras between C. glabrata Cnh1 and S. cerevisiae Nha1 Na+/H+-antiporters are functional proteins increasing the salt tolerance of yeast cells. Folia Microbiol 55, 435–441 (2010). https://doi.org/10.1007/s12223-010-0073-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-010-0073-y