Abstract
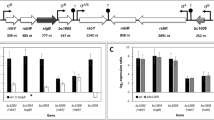
Stress proteomes of the cytoplasmic membrane fraction of Bacillus subtilis trp C2-exposed to acid pH and ethanol were characterized. Although these stress factors impair the cell function in a specific manner, they share the ability to denature proteins. Therefore, specific and general stress proteins in the membranes were investigated. Both ethanol (3 %) and pH 5.0 increase the doubling time from 17 to 25 min. Isolated cytoplasmic membranes were subjected to an optimized 2D PAGE analysis which permitted the separation and analysis of ≈450 distinct protein spots. Two alternative methods of protein detection were compared, i.e. silver staining and 35S-l-methionine pulse labeling; the stress induced proteins were identified by MALDI-TOF MS. After ethanol stress, five proteins were increased, viz. YdaP, Ctc, YfhM, YjcH and YwaC. Acid stress proteins were AcoB, YkwC, SodA, YjcH and YwaC. Proteins YjcH and YwaC were increased after ethanol as well as acid pH treatment.
Similar content being viewed by others
Abbreviations
- CHAPS:
-
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DTT:
-
1,4-dithiothreitol
- IPG:
-
immobilized pH gradient
- MALDI:
-
matrix-assisted laser desorption/ionization
- M :
-
molar mass
- MS:
-
mass spectrometry
- PAGE:
-
polyacrylamide gel electrophoresis
- PMSF:
-
phenylmethanesulfonyl fluoride
- SDS:
-
sodium dodecyl sulfate
- TOF:
-
time-of-flight
- µ:
-
growth rate
References
Ali N.O., Bignon J., Rapoport G., Debarbouille M.: Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J.Bacteriol. 183, 2497–2504 (2001).
Antelmann H., Scharf C., Hecker M.: Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J.Bacteriol.182, 4478–4490 (2000).
Betz C., Schlenstedt G., Bailer S.M.: Asr1p, a novel yeast ring/PHD finger protein, signals alcohol stress to the nucleus. J.Biol. Chem.279, 28174–28181 (2004).
Bisschop A., Konings W.N.: Reconstitution of reduced nicotinamide adenine dinucleotide oxidase activity with menadione in membrane vesicles from the menaquinone-deficient Bacillus subtilis AroD. Relation between electron transfer and active transport. Eur.J.Biochem.67, 357–365 (1976).
Cao M., Kobel P.A., Morshedi M.M., Wu M.F., Paddon C., Helmann J.D.: Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/ macroarray analysis (ROMA), and transcriptional profiling approaches. J.Mol.Biol.316, 443–457 (2002).
D’Amore T., Panchal C.J., Russel I., Stewart G.G.: A study of ethanol tolerance in yeast. Crit.Rev. Biotechnol.9, 287–304 (1990).
Halada P., Man P., Grebeňová D., Hrkal Z., Havlíček V.: Identification of HL60 proteins affected by 5-aminolevulinic acid-based photodynamic therapy using mass spectrometric approach. Collect.Czech.Chem.Commun.66, 1720–1728 (2001).
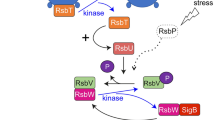
Gaidenko T.A., Price C.W.: General stress transcription factor σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J.Bacteriol.180, 3730–3733 (1998).
Gerner C., Vejda S., Gelbmann D., Bayer E., Gotzmann J., Schulte-hermann R., Mikulits W.: Concomitant determination of absolute values of cellular protein amounts, synthesis rates, and turnover rates by quantitative proteome profiling. Mol. Cell Proteomics1, 528–537 (2002).
Gharahdaghi F., Weinberg C.R., Meagher D.A., Imai B.S., Mische S.M.: Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis20, 601–605 (1999).
Grundy F.J., Waters D.A., Allen S.H., Henkin T.M.: Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J.Bacteriol.175, 7348–7355 (1993).
Hirose I., Sano K., Shioda I., Kumano M., Nakamura K., Yamane K.: Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology146, 65–75 (2000).
Huang X., Gaballa A., Cao M., Helmann J.D.: Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, sigma W. Mol.Microbiol.31, 361–371 (1999).
Inaoka T., Matsumura Y., Tsuchido T.: Molecular cloning and nucleotide sequence of the superoxide dismutase gene and characterization of its product from Bacillus subtilis. J.Bacteriol.180, 3697–3703 (1998).
Inaoka T., Matsumura Y., Tsuchido T.: SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J.Bacteriol.181, 1939–1943 (1999).
Jeong K.C., Hung K.F., Baumler D.J., Byrd J.J., Kaspar C.W.: Acid stress damage of DNA is prevented by Dps binding in Escherichia coli O157:H7. BMC Microbiol.15, 181 (2008).
Kaneda T.: Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol.Rev.55, 288–302(1991).
Malhotra L., Singh B.: Ethanol-induced changes in glycolipids of Saccharomyces cerevisiae. Appl.Biochem.Biotechnol.128, 205–213 (2006).
Marza E., Camougrand N., Manon S.: Bax expression protects yeast plasma membrane against ethanol-induced permeabilization. FEBS Lett.521, 47–52 (2002).
Nanamiya H., Kasai K., Nozawa A., Yun C.S., Narisawa T., Murakami K., Natori Y., Kawamura F., Tozawa Y.: Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol.Microbiol.67, 291–304 (2008).
Periago P.M., Van Schaik W., Abee T., Wouters J.A.: Identification of proteins involved in the heat stress response of Bacillus cereus ATCC 14579. Appl.Environ.Microbiol.68, 3486–3495 (2002).
Petersohn A., Bernhardt J., Gerth U., Höper D., Koburger T., Völker U., Hecker M.: Identification of sigma(B)-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J.Bacteriol.181,5718–5724 (1999).
Petersohn A., Brigulla M., Haas S., Hoheisel J.D., Völker U., Hecker M.: Global analysis of the general stress response of Bacillus subtilis. J.Bacteriol.183, 5617–5631 (2001).
Poolman B., Spitzer J.J., Wood J.M.: Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim.Biophys.Acta1666, 88–104 (2004).
Presser K.A., Ratkowsky D.A., Ross T.: Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl.Environ.Microbiol.63, 2355–2360 (1997).
Price C.W.: Protective function and regulation of the general stress response of Bacillus subtilis and related Gram-positive bacteria, pp. 179–197 in G. Storz, R. Hengge-Aronis (Eds): Bacterial Stress Responses. ASM Press, Washington (DC) 2000.
Price C.W.: General stress response, pp. 369–384 in A.L. Sonenshein, J.A. Hoch, R. Losick (Eds): Bacillus subtilis and its closest relatives. From genes to cells. ASM Press, Washington (DC) 2002.
Price C.W., Fawcett P., Ceremonie H., Su N., Murphy C.K., Youngman P.: Genome-wide analysis of the general stress response in Bacillus subtilis. Mol.Microbiol.41, 757–774 (2001).
Schmalisch M., Langbein I., Stülke J.: The general stress protein Ctc of Bacillus subtilis is a ribosomal protein. J.Mol.Microbiol.Biotechnol.4, 495–501 (2002).
Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C.: Measurement using bicinchoninic acid; elimination of interfering substances. Analyt.Biochem.180, 136–139 (1985).
Sonenshein A.L.: Bacterial sporulation: a response to environmental signals, pp. 199–215 in G. Storz, R. Hengge-Aronis (Eds): Bacterial Stress Responses. ASM Press, Washington (DC) 2000.
Takahashi T., Shimoi H., Ito K.: Identification of genes required for growth under ethanol stress using transposon mutagenesis in Saccharomyces cerevisiae. Mol.Genet.Genomics265, 1112–1119 (2001).
Thackray P.D., Moir A.: SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. J.Bacteriol.185, 3491–3498 (2003).
Völker U., Engelmann S., Maul B., Riethdorf S., VÖLKER A., Schmid R., Mach H., Hecker M.: Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology140, 741–752 (1994).
Völker U., Maul B., Hecker M.: Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J.Bacteriol.181, 3942–3948 (1999).
Wipat A., Harwood C.R.: The Bacillus subtilis genome sequence: the molecular blueprint of a soil bacterium. FEMS Microbiol.Ecol.28, 1–9 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petráčková, D., Šemberová, L., Halada, P. et al. Stress proteins in the cytoplasmic membrane fraction of Bacillus subtilis . Folia Microbiol 55, 427–434 (2010). https://doi.org/10.1007/s12223-010-0072-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-010-0072-z