Abstract
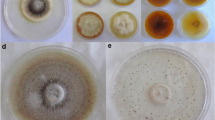
The pathogenicity of Ganoderma boninense was tested on coconut seedlings under greenhouse conditions and infection confirmed by using immunological and molecular diagnostic tools. Desiccation of older leaves and the emergence of sporophores were observed from pathogen-inoculated seedlings, whereas a control seedling does not show any pathogenic symptoms. Mature sporophores were formed within 10–13 weeks after inoculation. Polyclonal antibodies raised against mycelial proteins of Ganoderma were used for detection of Ganoderma in infected field palm and seedlings through indirect enzyme-linked immunosorbent assay technique. We adopted dot-immunobinding assay for the detection of Ganoderma from greenhouse and field samples. Under nucleic-acid-based diagnosis, G. boninense (167 bp) was detected from artificially inoculated seedlings and infected field palms by polymerase chain reaction. Apart from these, histopathological studies also support the Ganoderma pathogenicity in coconut seedlings. The pathogenicity test and combination of all the three diagnostic methods for Ganoderma could be highly reliable, rapid, sensitive and effective screening of resistance in planting material in the future.
Similar content being viewed by others
Abbreviations
- BSR:
-
basal stem rot
- PCR:
-
polymerase chain reaction
- DIBA:
-
dot-immunobinding assay
- PDA:
-
potato dextrose agar (broth)
- rDNA:
-
ribosomal DNA
References
Anonymous: All India co-ordinated research project on palms, progress report 1986–87. Centre of Plantation Crops Research Institute, Kasaragod (India) 1987.
Ariffin D., Idris A.S., Abdul Halim H.: Histopathological studies on colonization of oil palm root by Ganoderma boninense. Elaeis 3, 289–293 (1991).
Bhaskaran R., Ramanathan T., Ramiah M.: The Thanjavur wilt. Intens.Agric. 20, 19–21 (1982).
Bunting T.E., Plumley K.A., Clarke B.B., Hillman B.I.: Identification of Magnaporthe poae by PCR and examination of its relationship to other fungi by analysis of their nuclear rDNA ITS-1 region. Phytopathology 86, 398–404 (1996).
Darmono T.W.: Ganoderma in oil palm in Indonesia: current status and prospective use of antibodies for the detection of infection, pp. 249–266 in J. Flood, P.D. Bridge, M. Holderness (Eds): Ganoderma Diseases of Perennial Crops. CAB International, Oxon 2000.
Darus A., Seman I.A., Khairudin H.: Confirmation of Ganoderma infected palm by drilling technique, pp. 735–738 in PORIM International Palm Oil Congress: Update and Vision (Agriculture), Kuala Lumpur (Malaysia) 1993.
Gomez K.A., Gomez A.A.: Statistical Procedure for Agricultural Research. John Wiley and Sons, New York 1984.
Hampton R., Ball E., De Boer S.: Serological Methods for Detection and Identification of Viral and Bacterial Plant Pathogen. APS Press, New York 1990.
Hobbs H.A., Reddy D.V.R., Rajeswari R., Reddy A.S.: Use of antigen coating and protein A coating ELISA procedures for detection of three peanut viruses. Plant Dis. 71, 747–749 (1987).
Johansen D.A.: Plant Microtechnique. McGraw-Hill, New York 1940.
Moller E.M., Bahnweg G., Sanderman N.H., Geiger H.: A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies and infected plant tissues. Nucl.Acids Res. 20, 6115 (1992).
Moncalvo J.M., Wang H.H., Hseu R.S.: Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87, 223–238 (1995).
Natarajan S., Bhaskaran R., Shanmugam N.: Preliminary studies to develop techniques fir early detection of Thanjavur wilt in coconut. Ind.Coconut J. 17, 3–6 (1986).
Pearce R.B., Woodward S.: Compartmentalization and reaction zone barriers at the margin of decayed sapwood in Acer saccharinum L. Physiol.Mol.Plant Pathol. 29, 197–216 (1986).
Samiyappan R., Bhaskaran R., Rethinam P.: Diagnosis for early detection of Ganoderma diseases in perennial crops: approaches and prospects. J.Plant Dis.Protect. 103, 85–93 (1996).
Shanmugam V., Viswanathan R., Raguchander T., Balasubramanian P., Samiyappan R.: Immunology of the pathogen virulence and phytotoxin production in relation to disease severity: a case study in sheath blight of rice. Folia Microbiol. 47, 542–549 (2002).
Turner P.D.: Oil Palm Diseases and Disorders. Oxford University Press, Oxford 1981.
Viswanathan R., Samiyappan R., Padmanaban P.: Specific detection of Colletotrichum falcatum in sugarcane by serological technique. Sugarcane 3, 18–23 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandan, A., Radjacommare, R., Ramanathan, A. et al. Molecular biology of Ganoderma pathogenicity and diagnosis in coconut seedlings. Folia Microbiol 54, 147–152 (2009). https://doi.org/10.1007/s12223-009-0022-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-009-0022-9