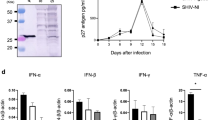
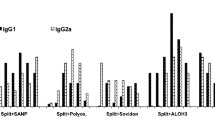
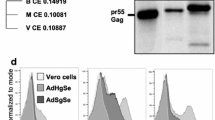
Abstract
Balb/c mice were immunized with the recombinant fusion protein gD1/313 (FpgD1/313 representing the ectodomain of HSV-1 gD), with the non-pathogenic ANGpath gE-del virus, with the plasmid pcDNA3.1-gD expressing full-length gD1 and with the recombinant immediate early (IE) HSV-1 protein ICP27. Specific antibodies against these antigens (as detected by ELISA) reached high titers with the exception of the DNA vaccine. High-grade protection against challenge with the virulent strain SC16 was found following immunization with the pcDNA3.1-gD plasmid and with the gE-del virus. Medium grade, but satisfactory protection developed after immunization with the FpgD1/313 and minimum grade protection was seen upon immunization with the IE/ICP27 polypeptide. A considerable response of peripheral blood cells (PBL) and splenocytes in the lymphocyte transformation test (LTT) was found in mice immunized with FpgD1/313, with the pcDNA3.1-gD plasmid and with the live ANGpathgE-del virus. For lymphocyte stimulation in vitro, the FpgD1/313 antigen was less effective than the purified gD1/313 polypeptide (cleaved off from the fusion protein); both proteins elicited higher proliferation at the 5 μg per 0.1 mL dose than at the 1 μg per 0.1 mL dose. The secretion of Th type 1 (TNF, IFN-γ and IL-2) and Th type 2 (IL-4 and IL-6) cytokines was tested in the medium fluid of purified PBL and splenocyte cultures; their absolute values were expressed in relative indexes. The PBL from FpgD1/313 immunized mice showed increased secretion of both TH1 (TNF) as well as TH2 (IL-4) cytokines (7–10-fold, respectively). Splenocytes from FpgD1/313 immunized mice showed a significant (23-fold) increase in IL-4 production.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- ConA:
-
concanavalin A
- DMEM:
-
Dulbecco minimal essential medium
- ELISA:
-
enzyme-linked immosorbent assay
- FCS:
-
fetal calf serum
- FLgD:
-
full-length glycoprotein D
- FpgD1/313:
-
fusion protein gD1/313 (313 amino acid ectodomain)
- gB:
-
glycoprotein B
- gC:
-
glycoprotein C
- gD:
-
glycoprotein D
- gE:
-
glycoprotein E
- gG:
-
glycoprotein G
- gH:
-
glycoprotein H
- HSV:
-
herpes simplex virus (human herpes virus)
- Hve:
-
herpesvirus entry mediator
- IF:
-
immunofluorescence
- IFN:
-
interferon
- IL:
-
interleukin
- LD50 :
-
lethal dose 50%
- LTT:
-
lymphocyte transformation test
- MoAb:
-
monoclonal antibody
- PBL:
-
peripheral blood leukocytes
- PBS:
-
phosphate-buffered saline
- PFU:
-
plaque-forming unit
- PHA:
-
phytohemagglutinin
- SI:
-
stimulation index
- TNF:
-
tumor necrosis factor
References
Aurelian L, Smith C.C., Wachsman M., Paoletti E.: Immune responses by herpes simplex virus in guinea pigs (footpad model) and mice immunized with vaccinia virus recombinants containing herpes simplex virus glycoprotein D. Rev.Infect.Dis. 13, 924–934 (1991).
Biron C.A., Sen G.C.: Interferons and other cytokines, pp. 321–351 in D.M. Knipe, P.M. Howley (Eds): Fields Virology. 4th ed. Lippincott-Williams and Wilkins, Philadelphia 2001.
Blacklaw B.A., Nash A.A.: Immunological memory to herpes simplex virus type 1 glycoproteins B and D in mice. J.Gen.Virol. 71, 863–871 (1990).
Bosch D.L., Geerligs H.J., Weijer W.J., Feijlbrief M., Welling G.W., Welling-Wester S.: Structural properties and reactivity of N-terminal synthetic peptides of herpes simplex virus type 1 glycoprotein D by using antipeptide antibodies and group VII monoclonal antibodies. J. Virol. 61, 3607–3611 (1987).
Broberg E.K., Peltoniemi J., Nygärdas M., Vahlberg T., Roytta M., Hukkanen V.: Spread and replication of and immune response to herpes simplex virus type vectors in Balb/c mice. J.Virol. 78, 13139–13152 (2004a).
Broberg E.K., Salmi A.A., Hukkanen V.: IL-4 is the key regulator in herpes simplex virus-based gene therapy of Balb/c experimental autoimmune enchalomyelitis. Neurosci.Lett. 364, 173–178 (2004b).
Broker M., Abel K.J., Kohler R., Hilfenhaus J., Amann E.: Escherichia coli-derived envelope protein gD but not gC antigens of herpes simplex virus protect mice against a lethal challenge with HSV-1 and HSV-2. Med.Microbiol.Immunol. 179, 145–149 (1990).
Bourne N., Milligan G.N., Schleiss M.R., Bernstein D.I., Stanberry L.R.: DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine 14, 1230–1234 (1996).
Bourne N., Bravo F.J., Francotts M., Bernstein D.I., Myers M., Slioui M., Stanberry L.: Herpes simplex virus type 2 glycoprotein D subunit vaccines and protection against genital HSV 1 and HSV 2 disease in guinea pigs. J.Infect.Dis. 187, 542–548 (2003).
Burke R.L., Goldbeck C., Ng P., Stanberry L., Ott G., Van Nest G.: The influence of adjuvant on the therapeutic efficacy of a recombinant genital herpes vaccine. J.Infect.Dis. 170, 1110–1119 (1994).
Cocchi F., Menom L., Dubreuil P., Lopez M., Campadelli-Fiume G.: Cell to cell spread of wild type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin 1 (PRRR1/HveC/HIgR) and nectin 2 (PRR2/HveB). J.Virol. 74, 3909–3917 (2000).
Cohen G.H., Isola V.J., Kuhns J., Berman P.W., Eisenberg R.J.: Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a non-denaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J.Virol. 60, 157–166 (1987).
Eisenberg R.J., Long D., Ponce L.M., Matthews J.T., Spear P.G., Gibson M.G., Lasky L.A., Berman P., Golub E., Cohen G.H.: Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J.Virol. 53, 634–644 (1985).
Ellermann-Eriksen S.: Macrophages and cytokines in the early defense against herpes simplex virus. Virology J. 2, 59 (2005).
Evans I.A.C., Jones C.A.: HSV induces an early primary TH1 CD4 T cell response in neonatal mice, but reduce CTL activity at the time of the peak response. Eur.J.Immunol. 35, 1454–1462 (2005).
Fló J.: Co-immunization with plasmids coding the full length and a soluble form of glycoprotein D of HSV-2 induces protective cellular and humoral immune response in mice. Vaccine 21, 1239–1245 (2003).
Fló J., Beatriz Perez A., Tisminetzky S., Baralle F.: Superiority of intramuscular route and full length glycoprotein D for DNA vaccination against herpes simplex 2. Enhancement of protection by the co-delivery of the GM-CSF gene. Vaccine 18, 3242–3253 (2000).
Fuller O.A., Spear P.G.: Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc.Nat.Acad.Sci.USA 84, 545–548 (1987).
Gyotoku T., Ono F., Aurelian L.: Development of HSV-specific CD4+ TH1 responses and CD8+ cytotoxic T lymphocytes with antiviral activity by vaccination with the HSV-2 mutant ICP10delPK. Vaccine 20, 2796–2807 (2002).
Harari A., Vallelian F., Meylan P.R., Pantolen G.: Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J.Immunol. 174, 1037–1045 (2005).
Halford W.P., Gebhardt B.M., Carr D.J.J.: Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J.Immunol. 157, 3542–3549 (1996).
Harandi A.M., Svennweholm B., Holmgren J., Eriksson K.: Differential roles of B cells and IFN-γ secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J.Gen. Virol. 82, 845–853 (2001).
Higgins T.J., Herold K.M., Arnold R.L., McElhiney S.P., Shroff K.E., Pachuk C.J.: Plasmid DNA-expressed secreted and non-secreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J.Infect.Dis. 182, 1311–1320 (2000).
Highlander S.L., Sutherland S.L., Gage P.J., Johnson D.C., Levine M., Glorioso J.C.: Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit penetration. J.Virol. 61, 3356–3364 (1987).
Hukkanen V., Bromberg E., Salmi A., Eralinna J.P.: Cytokines in experimental herpes simplex virus infection. Internat.Rev.Immunol. 21, 355–371 (2002).
Inoue T., Inoue Y., Nakamura Y., Yoshida A., Inoue Y., Tano Y., Shimomura Y., Fujisawa Y., Aono A., Hayashi K.: The effect of immunization with herpes simplex virus glycoprotein D fused with interleukin 2 against murine herpetic keratitis. Japan.J.Ophthalm. 46, 370–376 (2001).
Košovský J., Ďurmanová V., Kúdelová M., Režuchová I., Tkáčiková L’., Rajčáni J.: A simple procedure for expression and purification of selected non-structural (α and β) herpes simplex virus 1 proteins. J.Virol.Meth. 92, 121–129 (2000).
Krug A., Luker G.D., Barchet W., Leib D.A., Akira C., Colonna M.: Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103, 1433–1437 (2004).
Lasky L.A., Dowbenko D., Simmonsen C.C., Berman P.W.: Protection of mice from lethal herpes simplex virus infection by vaccination with a secreted form of cloned glycoprotein D. BioTechnology 2, 527–532 (1984).
Lee H.H., Cha S.C., Jang D.J., Lee J.K., Choo D.W., Kim Y.S., Uh H.S., Kim S.Y.: Immunization with combined HSV-2 glycoproteins B2:D2 gene DNAs: protection against lethal intravaginal challenges in mice. Virus Genes 25, 179–188 (2002).
Liu T., Khanna K.M., Chen X.P., Fink D.J., Hendricks R.L.: CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J.Exp.Med. 191, 1459–1466 (2000).
Long D., Wilcox W.C., Abrams W.R., Cohen G.H., Eisenberg E.J.: Disulfide bond structure of glycoprotein D of herpes simplex virus types 1 and 2. J.Virol. 66, 6668–6685 (1992).
Malmgaard L., Paluidan S.R., Mogensen S.C., Ellerman-Ericksen S.: Herpes simplex virus induces selection of IL-12 by macrophages through a mechanism involving NFκB. J.Gen.Virol. 81, 3011–3020 (2000).
Malmgaard L., Paludan S.R.: Interferon α/β, interleukin 12 and IL-18 induce production of IFN-γ during infection with herpes simplex virus type 2. J.Gen.Virol. 84, 2497–2500 (2003).
Mannickan E., Francotte M., Kuklin N., Dewerchin M., Molitor C., Gheysen D., Slaoui M., Rouse B.T.: Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+-TH1 cells. J.Virol. 69, 4711–4716 (1995).
Manservigi R., Boero A., Argnani R., Caselli E., Zucchini S., Miriagou V., Mavromara P., Cilli M., Grossi M.P., Balboni P.G., Cassai E.: Immunotherapeutic activity of a recombinant combined gB-gD-gE vaccme against recurrent HSV-2 infections in a guinea pig model. Vaccine 4, 865–872 (2005).
Martin S., Rouse B.T.: The mechanism of antiviral immunity induced by vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D clearance of local infection. J.Immunol. 138, 3431–3437 (1987).
McClements W.L., Armstrong M.E., Keys R.D., Liu M.A.: The prophylactic effect of immunization with DNA encoding herpes simplex virus glycoproteins on HSV-induced disease in guinea pigs. Vaccine 15, 857–860 (1997).
McNally J.M., Dempsey D., Wolcott R.M., Chervenak R., Jennings S.R.: Phenotypic identification of age-dependent VD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J.Immunol. 163, 675–681 (1999).
Minson A.C., Hodgman T.C., Digard P., Hancock D.C., Bell S.E., Buckmaster E.A.: An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J.Gen.Virol. 67, 1001–1013 (1986).
Mishkin E.M., Fahey J.R., Kino Y., Klein R.J., Abramovitz A.S., Mento S.J.: Native herpes simplex virus glycoprotein D vaccine: immunogenicity and protection in animal model. Vaccine 9, 147–153 (1991).
Mohamedi S.A., Heath A.W., Jennings H.R.: A comparison of oral and parenteral routes for therapeutic vaccination with HSV-2 ISCOM in mice: cytokine profiles, antibody responses and protection. Antiviral Res. 49, 83–99 (2001).
Morrison L.A., Knipe D.M.: Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 230, 402–413 (1996).
Morrison L.A., Zhu L., Thebau L.G.: Vaccine induced serum immunoglobulin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J.Virol. 75, 1195–1204 (2001).
Moško T., Košovský J., Režuchová I., Ďurmanová V., Kúdelová M., Rajčáni J.: Expression of herpes simplex virus glycoprotein D in prokaryotic and eukaryotic cells. Acta Virol. 48, 97–107 (2004).
Muggeridge M.I., Wu T.T., Johnson D.C., Glorioso J.C., Eisenberg R.J., Cohen G.H.: Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology 174, 375–387 (1990).
Myers M.G., Bernstein D., Harrison C.J., Stanberry L.R.: Herpes simplex virus glycoprotein treatment of recurrent genital herpes reduces cervicovaginal virus shedding in guinea pigs. Antiviral.Res. 10, 83–88 (1988).
Nass P.H., Elkins K.L., Weir J.P.: Protective immunity against herpes simplex virus generated by DNA vaccination compared to natural infection. Vaccine 19, 1538–1546 (2001).
Neidhardt H., Schroder C.H., Kaerner H.C.: Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J.Virol. 61, 600–603 (1987).
Osorio Y., Ghiasi H.: Comparison of adjuvant efficacy of herpes simplex virus type 1 recombinant viruses expressing TH1 and TH2 cytokine genes. J.Virol. 77, 5774–5783 (2003).
Rajčáni J., Ďurmanová V.: Developments in herpes simplex virus vaccines: old problems and new challenges. Folia Microbiol. 51, 67–85 (2006).
Rajčáni J., Sabó A., Mucha V., Košťál M., Compel P.: Herpes simplex virus type 1 envelope subunit vaccine not only protects against lethal virus challenge, but also may restrict latency and virus reactivation. Acta Virol. 39, 37–49 (1995).
Rajčáni J., Vojvodová A., Matis J., Kúdelová M., Dragúňová J., Krivjanská M., Zelník V.: The syn3 strain of herpes simplex virus is not pathogenic for mice and shows limited neural spread. Virus Res. 41, 33–44 (1996).
Rajčáni J., Moško T., Režuchová I.: Current developments in viral DNA vaccines: shall they solve the unsolved? Rev.Med.Virol. 15, 303–325 (2005).
Reed L. J., Muench H.: A simple method of estimating 50 per cent end-points. Amer.J.Hyg. 27, 493–497 (1938).
Rooney J.F., Wohlenberg C.H., Cremer K.J., Moss B., Notkins A.L.: Immunization with a vaccinia virus recombinant expressing herpes simplex virus glycoprotein D: long-term protection and effect of revaccination. J.Virol. 62, 1530–1534 (1988).
Rosenthal K.L., Smiley J.R., South S., Johnson D.C.: Cells expressing herpes simplex virus glycoprotein C but not gB, gD or gE are recognized by murine virus specific cytotoxic T lymphocytes. J.Virol. 61, 2438–2447 (1987).
Rouse B.T., Nair S., Rouse R.J., Yu Z., Kuklin N., Karem K., Manickan E.: DNA vaccines and immunity to herpes simplex virus. Curr.Topics Microbiol.Immunol. 226, 69–78 (1998).
Rouse B.T., Sarangi P.P., Suvas S.: Regulatory T cells in virus infections. Immunol.Rev. 212, 272–286 (2006).
Sin J.I., Kim J.J., Arnold R.L., Shroff K.E., McCallus D., Pachuk C., McElhiney S.P., Wolf M.W., Pompa-de Bruin S.J., Higgins T.J., Ciccarelli R.B., Weiner D.B.: IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances TH1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J.Immunol. 162, 2912–2921 (1999).
Singh M., Carlson J.R., Briones M.: A comparison of biodegradable microparticles and MF59 as systematic adjuvants for recombinant gD from HSV 2. Vaccine 16, 1822–1827 (1998).
Stanberry L.R., Cunningham A.L., Mindel A., Scott L.L., Spruance S.L., Aoki F.Y., Lacey C.J.: Prospects for control of herpes simplex virus disease through immunization. Clin.Infect.Dis. 30, 549–566 (2000a).
Stanberry L.R.: Genital and perinatal herpes simplex virus infections: prophylactic vaccines, pp. 187–216 in L.R. Stanberry, D.I. Bemstein (Eds): Sexually Transmitted Diseases. Vaccines, Prevention and Control. Academic Press, London 2000b.
Thomas J., Kanangat S., Rouse R.T.: Herpes simplex virus replication induced expression of chemokines and proinflammatory cytokines in the eye: implication in herpetic stromal keratitis. J.Interferon Cytokine Res. 18, 681–690 (1998).
Vandepapeliere P.: Therapeutic vaccines for control of herpes simplex virus chronic infections, pp. 217–238 in L.R. Stanberry, D.I. Bernstein (Eds): Sexually Transmitted Diseases. Vaccines, Prevention and Control. Academic Press, London 2000.
Wachsman M., Luo J.H., Aurelian L., Perkus M.E., Paoletti E.: Antigen presenting capacity of epidermal cells infected with vaccinia virus recombinants containing the herpes simplex virus glycoprotein D and protective immunity. J.Gen.Virol. 70, 2513–2520 (1989).
Whitbeck J.C.H., Peng C.H., Lou H., Xu R., Willis S.H., Ponce de Leon M., Peng T., Nicola A.V., Mongomery R.I., Warner M.S., Soulika A.M., Spruce L.A., Moore W.T., Lambris J.D., Spear P.G., Cohen G.H., Eisenberg R.J.: Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of tumor necrosis factor receptor subfamily and mediator of HSV entry. J.Virol. 71, 6083–6093 (1997).
York I.A., Giorgio D.P., Mishkin E.M.: Immunomodulatory effects of HSV-2 glycoprotein D in HSV/1/infected mice: implications for immunotherapy of recurrent HSV infection. Vaccine 13, 1706–1712 (1995).
Zheng M., Atherton S.: Cytokine profiles and inflammatory cells during HSV-1 induced acute retinal necrosis. Invest. Ophthalmol. Vis.Sci. 46, 1356–1363 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ďurmanová, V., Sapák, M., Košovský, J. et al. Immune response and cytokine production following immunization with experimental herpes simplex virus 1 (HSV-1) vaccines. Folia Microbiol 53, 73–83 (2008). https://doi.org/10.1007/s12223-008-0011-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-008-0011-4