Abstract
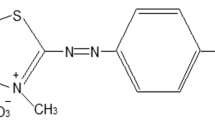
The adsorption is widely used to remove certain classes of pollutants from water, especially those that are hardly biodegradable and dyes represent one of these problematic groups. The removal of bromothymol blue (BTB) from wastewater using TiO2 was studied in batch system. The adsorbent TiO2 has a specific surface area of 400 m2/g, a mean crystallites sizes (5–10 nm), and pHpzc equal to 6.5. TiO2 is stable over the whole pH range and constitutes a good compromise between efficiency and stability (in both acidic and basic media), therefore, the use of other additives is not necessary. Its non-toxicity and low energy required for its activation (E ~ 3 eV) as well as its low cost for most of the applications envisaged make it advantageous. The influence of effective variables such as solution pH (1–10), contact time (0–60 min), initial BTB concentration (5–40 mg/l), adsorbent dose of TiO2 (0.2–2 g/l), and temperature (20–60 °C) on the adsorption efficiency was examined, while the BTB content was determined by UV–Vis spectrophotometry. The optimal pH, adsorbent dose, and contact time for the efficient removal were found to be 10, 0.2 g/l, and 30 min, respectively, and the adsorbent was characterized by the BET analysis and point of zero charge (pHpzc). Among the different kinetic models, the experimental data of the BTB removal are well fitted with the pseudo-first-order kinetic model with a high determination coefficient. The evaluation of the fitness of equilibrium data by various conventional isotherm models, based on the R2 value as criterion, show the successful applicability of the Langmuir model for the interpretation of experimental data with a maximum adsorption capacity (qmax) of 27.02 mg/g at 20 °C and R2 of 0.997. The adsorption isotherms at different temperatures have been used for the determination of the free energy (ΔGo = 2.1808 to—1.0981 kJ/mol), enthalpy (ΔHo = 20.74 kJ/mol), and entropy (ΔSo = 65.58 J/mol/K) indicate that the overall adsorption is spontaneous and endothermic in nature.










Similar content being viewed by others
References
I. Anastopoulos, G.Z. Kyzas, J. Mol. Liq. 200, 381 (2014)
I. Ali, M. Asim, T.A. Khan, J. Environ. Manag. 113, 170 (2012)
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, J. Colloid, Interface Sci. 209, 172 (2014)
I. Kabdasli, O. Tunay, D. Orhon, Water Sci. Technol. 40, 261 (1996)
M. Mahramanlioglu, M. Zahoor, A. Cinarli, I. Kizilcikli, Fresenius Environ. Bull. 19, 911 (2010)
Y.C. Wong, Y.S. Szeto, W.H. Cheung, G. McKay, Process Biochem. 39, 695 (2004)
B. Saritha, K. Rajasekhar, K. Kiso, Int. J. Innov. Res. Sci, Eng. Technol., 4, 6821 (2015).
K.H. Hassan, E.M. Mahdi, Asian J. Appl. Sci. 4, 730 (2016)
V.K. Gupta, I. Ali, T.A. Saleh, A. Nayak, S. Agarwal, RSC Adv. 2, 6380 (2012)
I. Ali, T.A. Khan, M. Asim, Sep. Purif. Rev. 40, 25 (2011)
S. Karthikeyan, V.K. Gupta, R. Boopathy, A. Titus, G. Sekaran, J. Mol. Liq. 173, 153 (2012)
T.A. Saleh, V.K. Gupta, J. Colloid Interface Sci. 371, 101 (2012)
M. Abbas, M. Trari, Desalin. Water Treat. 180, 398 (2020)
M. Abbas, M. Trari, Scientific African 8, e00387 (2020)
Y.-H. Chiu, T.-F. Mark Chang, C.-Y. Chen, M. Sone, and Y.-J. Hsu, Catalysts, 9, 430 (2019).
M.-J. Fang, C.-W. Tsao, Y.-J. Hsu, J. Phys. D: Appl. Phys. 53, 143001 (2020)
Y.-H. Chiu, Y.-J. Hsu, Nano Energy 31, 286 (2017)
Y.C. Pu, H.-Y. Chou, W.-S. Kuo, K.-H. Wei, Y.-J. Hsu, Appl. Catal. B: Environ. 204, 21 (2017)
Y.C. Pu, W.-H. Lin, Y.-J. Hsu, Appl. Catal. B: Environ. 163, 343 (2015)
Y.-D. Chiou, Y.-J. Hsu, Appl. Catal. B: Environ. 105, 211 (2011)
Y.-F. Lin, Y.-J. Hsu, Appl. Catal. B: Environ. 130–131, 93 (2013)
M.-Y. Chen, Y.-J. Hsu, Nanoscale 5, 363 (2013)
R. Saravanan, E. Sacari, F. Gracia, M.M. Khan, E. Mosquera, V.K. Gupta, J. Mol. Liq. 221, 1029 (2016)
M. Ghaedi, S. Hajjati, Z. Mahmudi, I. Tyagi, S. Agarwal, A. Maity, V.K. Gupta, Chem. Eng. J. 268, 28 (2015)
S. Rajendran, M.M. Khan, F. Gracia, J. Qin, V.K. Gupta, S. Arumainathan, Sci. Rep. 6, 31641 (2016)
M. Abbas, Adsorpt. Sci. Technol. 38, 24 (2020)
M. Abbas, Z. Harrache, M. Trari, J. Eng. Fiber. Fabr. 15, 1 (2020)
M. Abbas, Mater. Today: Proc. 31, 437 (2020)
M. Abbas, J. Water Reuse Desalin 10, 251 (2020)
M. Abbas, M. Trari, Fibers Polym. 21, 810 (2020)
M. Abbas, Z. Harrache, M. Trari, Adsorpt. Sci. Technol. 37, 566 (2019)
E.A. Dil, M. Ghaedi, A.M. Ghaedi, A. Asfaram, A. Gourdarzi, S. Hajati, M. Soylak, S. Agarwal, V.K. Gupta, J. Ind. Eng. Chem. 34, 186 (2016)
Suhas, V. K. Gupta, P. J. M. Carrott, R. Singh, M. Chaudhary, and S. Kushwaha, Bioresour. Technol., 216, 1066 (2016).
A.E. Burakov, E.V. Galunin, I.V. Burakova, A.E. Kucherova, S. Agarwal, A.G. Tkachev, V.K. Gupta, Ecotoxicol. Environ. Saf. 148, 702 (2018)
X. Liu, S.Q. Zhang, X. Wei, T. Yang, M.L. Chen, J.H. Wang, Biosens. Bioelectron. 109, 150 (2018)
C. Rukchon, A. Nopwinyuwong, S. Trevanich, T. Jinkarn, P. Suppakul, Talanta 130, 547 (2014)
L. Gao, X. Yang, Y. Shu, X. Chen, J. Wang, J. Colloid Interface Sci. 512, 819 (2018)
M. Abbas, T. Aksil, M. Trari, Desalin. Water Treat. 125, 93 (2018)
S. Agarwal, H. Sadegh, M. Monajjemi, A.S. Hamdy, G.A.M. Ali, A.O.H. Memar, S. Ghoshekandi, R. Tyagi, V.K. Gupta, J. Mol. Liq. 218, 191 (2016)
V.K. Gupta, A. Mittal, V. Gajbe, J. Colloid Interface Sci. 284, 89 (2005)
W.-T. Tsai, H.-C. Hsu, T.-Y. Su, K.-Y. Lin, C.-M. Lin, T.-H. Dai, J. Hazard. Mater. 147, 1056 (2007)
Z. Harrache, M. Abbas, T. Aksil, M. Trari, Desalin. Water Treat. 147, 273 (2019)
T. Sauer, G. Cesconeto Neto, H. J. José, and R. F. Moreira, J. Photochem. Photobiol. A: Chem., 149, 147 (2002).
M.H. Habibi, A. Hassanzadeh, S. Mahdavi, J. Photochem. Photobiol. A: Chem. 172, 89 (2005)
Y. S. Ho and G. McKay, Trans IChemE, 76, Part B, 183 (1998).
R.S. Juang, M.L. Chen, Ind. Eng. Chem. Res. 36, 813 (1997)
G. Woodbury, Physical Chemistry (Brooks/Cole Publishing Company, New York, 1997), p.820
W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 89, 31 (1963).
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
M. Temkin, V. Pyzhev, Acta Physicochim. URSS 12, 327 (1940)
H.M.F. Freundlich, Z. Phys, Chem. 57, 385 (1906)
M. Ghaedj, F. Karimi, B. Barrazzch, R. Sahraei, A. Danichfar, J. Ind. Eng. Chem. 19, 756 (2013)
A. Ahmad, M. Rafatullah, O. Sulaiman, M.H. Ibrahim, R. Hashim, J. Hazard. Mater 170, 357 (2009)
M. Ghaedi, A. Ansari, M.H. Habibi, A.R. Asghari, J. Ind. Eng. Chem. 20(1), 17 (2014)
D. Robati, M. Rajabi, O. Moradi, F. Najafi, I. Tyagi, S. Agarwal, V.K. Gupta, J. Mol. Liq 214, 259 (2016)
T.A. Khan, M. Nazir, Environ. Prog. Sustain Energy 34, 1444 (2015)
M. Ghaedi, E. Nazari, R. Sahrai, M.K. Purkait, Desalin. Water Treat. 52, 5504 (2014)
S.I. Abubaker, M.B. Ibrahim, J. Pure Appl. Sci. 11, 273 (2018)
A.E. Adnan, T.A. Selman, Res. J. Pharm. Biol. Chem. Sci. 7, 591 (2016)
S.H. Lubbad, B.K.A. Al-Roos, F.S. Kodeh, Curr. Green Chem. 6, 53 (2019)
Acknowledgements
The financial support of the work was provided by Boumerdes University, Science faculty, Chemical department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors attest that there are no conflict of interest and financial, personal, or other relationships with other people, laboratories, or organizations worldwide.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbas, M. Potential of Titanium Dioxide to Remove Bromothymol Blue (BTB) in Aqueous Solution by Batch Mode Adsorption–Kinetic, Isotherm and Thermodynamic Studies. Fibers Polym 24, 603–612 (2023). https://doi.org/10.1007/s12221-023-00022-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-023-00022-0