Abstract
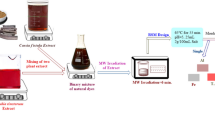
The aim of coloration for a textile material is to achieve high-quality dyed fabric with good levelness, fastness, and ecological and economical process. In this study, the potential use of molasses as an ecological reducing agent was evaluated for CI Leuco Sulfur Black 1 with respect to the redox potential values of the dyeing liquid, dyeability, fastness values, and waste liquid parameters. It was concluded that molasses can be used instead of commercial products in the exhaustion method and is easily applicable for bulk processes in dyeing mills. Furthermore, the color yield can be improved by increasing the absorption value and impregnation temperature and by using mercerized fabric in the pad-dyeing method.
Similar content being viewed by others
References
S. M. Burkinshaw and G. Salihu, Dyes Pigm., 161, 581 (2019).
S. M. Burkinshaw and G. Salihu, Dyes Pigm., 161, 614 (2019).
E. R. Trotman, “Dyeing and Chemical Technology of Textile Fibres”, 6th ed., pp.396–406, Oxford Verbatim Ltd., London, 1993.
J. N. Chakraborty, “Fundamentals and Practices in Colouration of Textiles”, 2nd ed., pp.46–58, Woodhead Publishing India PVT Ltd., New Delhi, 2014.
A. K. R. Choudhury, “Textile Preparation and Dyeing”, pp.585–597, Science Publishers, Enfield, New Hampshire, 2006.
C. Kan, H. Cheung, and F. Kooh, Fiber. Polym., 18, 767 (2017).
M. Mohsin, A. Rasheed, A. Farooq, M. Ashraf, and A. Shah, J. Cleaner Prod., 53, 341 (2013).
J. R. Aspland, Text. Chem. Color., 24, 21 (1992).
M. D. Teli, R. Paul, S. M. Landage, and A. Aich, Indian J. Fibres Text. Res., 26, 101 (2001).
H. Zollinger, “Color Chemistry Syntheses, Properties, and Applications of Organic Dyes and Pigments”, 3rd ed., pp.351–363, Wiley-VCH, Zürich, 2003.
M. Bozic and V. Kokol, Dyes Pigm., 76, 299 (2008).
Y. V. Polenov, V. A. Pushkina, V. V. Budanov, and O. S. Khilinskaya, Russ. J. Appl. Chem., 74, 1301 (2001).
G. Tchobanoglous and F. L. Burton, “Waste Water Engineering Treatment, Disposal and Reuse”, 3rd ed., pp.47–111, McGraw-Hill, Inc., 1991.
M. Clark, “Handbook of Textile and Industrial Dyeing”, Woodhead Publishing Ltd., Oxford, Cambridge, 2011.
H. Tanveer and A. Wahab, J. Cleaner Prod., 198, 806 (2018).
M. Teklehaimanot and T. Amsalu, J. Text. Sci. Eng., 8, 384 (2018).
R. S. Blackburn and A. Harvey, Environ. Sci. Technol., 38, 4034 (2004).
A. Madhu, G. Singh, A. Sodhi, S. Malik, and Y. Madotra, J. Text. Apparel Technol. Manag., 7, 1 (2012).
P. Jaruhar and J. N. Chakraborty, Text. Res. J., 83, 1345 (2013).
S. K. Nicholson and P. John, Appl. Microbiol. Biotechnol., 68, 117 (2005).
W. Czajkowski and J. Misztal, Dyes Pigm., 26, 771 (1994).
W. Marte and R. Ulisbach, U. S. Patent, 4950306 (1990).
A. Vuorema, P. John, M. Keskitalo, M. A. Kulandainathan, and F. Marken, Dyes Pigm., 76, 542 (2008).
T. Bechtold, A. Turcanu, H. Brunner, and W. Schrott, J. Appl. Electrochem., 39, 1963 (2009).
S. M. Burkinshaw and J. W. Collins, Dyes Pigm., 29, 323 (1995).
S. R. Shukla and R. S. Pai, Indian J. Fibres Text. Res., 29, 454 (2004).
N. A. Ibrahim, A. R. El-Gamal, and F. Mahrous, J. Nat. Fibres, 5, 238 (2008).
R. B. Chavan and S. Vhanbatte, Indian J. Fibres Text. Res., 27, 179 (2002).
K. Celik, MS Dissertation, SDU, Isparta, 2018.
M. F. Kavas and M. J. Leblebici, “Kalite ve Isletme Kontrol Laboratuvarlari El Kitabi”, Turkiye Seker Fabrikalari A.S., Genel Mudurlugu Ankara (In Turkish), pp.129–130 and p.246, 2004.
Archroma (www.archroma.com), Diresul Technical Information, pp.1–52, 2013.
Archroma (www.archroma.com), Leonil® EHC Liquid Conc. Technical Information, pp.1–3, 2013.
Archroma (www.archroma.com), Dekol® 2005 Liquid Conc., Technical Information, pp.1–5, 2013.
Archroma (www.archroma.com), Reducing Agent D p Technical Information, pp.1–4, 2013.
F. Akarslan, Ph.D. Dissertation, SDU, Isparta, 2008.
R. Veberic and F. Stampar, In Proceeding of the 2005 FRUTIC Conference, Montpellier, 2005.
Hach Company (www.hach.com)/Hach Lange GmbH, Sulfite DOC316. 53.01162, Iodate-iodide Method, Method 8071, 0–500 mg/L as SO3−2 (or 0 to more than 500 mg/L), Buret Titration, 2007–2015.
Hach Company (www.hach.com)/Hach Lange GmbH, Sulfite DOC316.52. 93090, Based on 4500-SO3−2 B in Standard Methods for the Examination of Water and Wastewater, Amperometric Back Titration, 2014–2016.
R. Zouhaier, D. Sofiène, and S. Faouzi, Eur. Sci. J., 10, 436 (2014).
J. M. Carraher, C. N. Fleitman, and J. P. Tessonnier, ACS Catal., 5, 3162 (2015).
S. Zhao, X. Guo, P. Bai, and L. V. Lingjuan, Asian J. Chem., 26, 4537 (2014).
T. Bechtold, F. Berktoldand, and A. Turcanu, Color. Technol., 116, 215 (2000).
L. A. Meszaros, U. S. Patent, 5470356 (1995).
A. F. Carey, “Organic Chemistry”, 2nd ed., McGraw-Hill, Inc., New York, 1987.
Y. Yang and V. Naarani, Color. Technol., 120, 127 (2004).
S. Fu, D. Hinks, P. Hauser, and M. Ankeny, Cellulose, 20, 3101 (2013).
S. R. Karmakar, “Chemical Technology in the Pre-Treatment Processes of Textiles”, pp.279–309, Elsevier Publishing, Amsterdam, 1999.
M. A. Kulandainathan, K. Patil, A. Muthukumaran, and R. B. Chavan, Color. Technol., 123, 143 (2007).
L. Saikhaoa, J. Setthayanond, T. Karpkird, T. Bechtold, and P. Suwanruji, J. Cleaner Prod., 197, 106 (2018).
Archroma, Reducing Agent D Powder, MSDS, KS13599, pp.1–11, 2018.
Acknowledgements
The authors are particularly grateful to Mahir Torsun and Hamza Kılıc (Kadifeteks Corporation, İstanbul) for color evaluation, and Ufuk Elibuyuk for his help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bulut, M.O., Çelik, K. Evaluation of Molasses as a Green Reducing Agent in Sulfur Dyeing. Fibers Polym 21, 2024–2035 (2020). https://doi.org/10.1007/s12221-020-1231-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-020-1231-8