Abstract
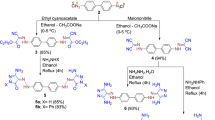
N4-(5-methyl-1,3,4-thiadiazol-2-yl)thiazole-2,4-diamine derivatives have been synthesized by the reaction of 2-amino-5-methyl-1,3,4-thiadiazole and chloroaecetylchloride with suitable solvent, then cyclized with thiourea at reflux temperature in methanol to yield N4-(5-methyl-1,3,4-thiadiazol-2-yl)thiazole-2,4-diamine which was diazotized and coupled with various naphthalene acid couplers to give new series of acid dyes (AD1-AD13). All the compounds were characterized by their percentage yield, melting point, elemental analysis, UV spectra, IR spectra, and NMR spectra and dyeing performance on nylon fabric has been assessed.
Similar content being viewed by others
References
(a) B. W. Gung and R. T. Taylor, J. Chem. Ed., 81, 1630 (2004).
C. Decelles, J. Chem. Ed., 26, 583 (1949).
S. C. Catino and E. Farris, “Concise Encyclopedia of Chemical Technology”, John Wiley & Sons, New York, 1985.
K. Venkatraman, “The Chemistry of Synthetic Dyes”, 3rd ed., p. 249, New York, 1970.
D. M. Lewis, “Wool Dying”, Society Dyers and Colourists, p. 283, 1992.
N. B. Patel and A. L. Patel, Asian J. Chem., 21, 4435 (2009).
N. B. Patel and A. L. Patel, Oriental J. Chem., 24, 551 (2008).
N. B. Patel and A. L. Patel, Indian J. Chem., 48B, 705 (2009).
D. N. Naik and K. R. Desai, Dyes Pigm., 14, 1 (1990).
F. Raffi, J. D. Hall, and C. E. Cernigila, Food Chem. Toxicol., 35, 897 (1997).
N. Parekh, K. Maheria, and P. Patel, J. Sci. Ind. Res, 70, 525 (2011).
B. C. Dixit, H. M. Patel, R. B. Dixit, and D. J. Desai, J. Serb. Chem. Soc., 75, 605 (2010).
S. H. Yoon, T. K. Kim, Y. J. Lim, and Y. A. Son, J. Korean Soc. Dyers Finishers, 14, 35 (2002).
O. Annen, R. Egli, R. Hasler, B. Henzi, H. Jakob, and P. Matzinger, Rev. Prog. Colouration, 17, 72 (1987).
H. Berneth, U. Claussen, W. Hartwich, and P. Wild, Eur. Pat. Appl., EP 675169 (1996).
H. Berneth, W. Hartwich, and K. H. Lange, Ger. Offen., DE 4222257 (1994).
Y. Ishida and J. Murata, Jpn. Kokai Tokkyo Koho, JP 08113722 (1996).
H. Berneth and H. Giera, Eur. Pat. Appl., EP 717082 (1996).
A. P. Shawcross, R. Bradbury, B. H. Meyrick, and M. Hobrook, PCT Int. Appl., WO 9950357 (1998).
H. R. Maradiya, Chem. Heterocycl. Comp., 45, 1252 (2009).
F. Hadizadeh and R. Vosoogh, J. Heterocycl. Chem., 45, 1 (2008).
S. M. Lu and R. Y. Chen, Org. Prep. Proced. Int., 32, 302 (2000).
R. M. El-Shishtawy, Int. J. Photoenergy, 2009, 1 (2009).
J. Barber and B. Andersson, Nature, 370, 31 (1994).
H. R. Maradiya and V. S. Patel, Fiber. Polym., 2, 212 (2001).
H. R. Maradiya, J. Serb. Chem. Soc., 67, 709 (2002).
S. K. Patel, P. K. Patel, and G. M. Malik, IOSR-JAC, 7, 8 (2014).
S. K. Sonwane, S. D. Srivastava, and S. K. Srivastava, Indian J. Chem., 47B, 633 (2008).
T. Singh, S. Sharma, V. K. Srivastava, and A. Kumar, Indian J. Chem., 45B, 1557 (2006).
G. M. Malik, P. C. Patel, J. H. Tailor, and S. K. Zadafiya, IRJNAS, 1, 108 (2014).
E. B. Towne, J. B. Dickey, and M. S. Bloom, U. S. Patent, 2852504 (1958).
N. Parekh and K. C. Maheria, Indian J. Fibre Text. Res., 37, 372 (2012).
A. Szymczyk, N. Fatin-Rouge, P. Fievet, C. Ramseyer, and A. Vidonne, J. Membr. Sci., 287, 102 (2007).
G. Hallas and J. H. Choi, Dyes Pigm., 42, 249 (1999).
A. K. Bello, Dyes Pigm., 27, 45 (1995).
Standard Test Method BS 1006, “Colour Fastness Standards for Textiles and Leather”, 1978, UK: ISO 105, 1995.
M. Freund and C. Meinecke, Bes. Dtsch. Chem. Ges., 29, 2511 (1896).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Malik, G.M., Patel, P.C., Tailor, J.H. et al. Synthesis, Characterization, and Dyeing Performance of Thiadiazole Derivatives. Fibers Polym 19, 1670–1677 (2018). https://doi.org/10.1007/s12221-018-7310-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-018-7310-4