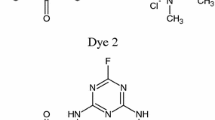
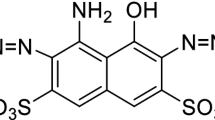
Abstract
Reactive dyes are used widely in cotton dyeing process. Reactive dyeing uses high amounts of inorganic salts to accelerate dye exhaustion. These salts are then discharged to the effluent, leading to serious environmental problems. Biodegradable organic salts can alternatively be used in the dyeing process. In this paper, a new liquid organic salt was synthesized by acid-base neutralization, which was used to replace inorganic salts in reactive dyeing. Dye exhaustion in organic salt dyeing was similar to the values in conventional dyeing. However, dye fixations were slightly lower in organic salt dyeing than in conventional dyeing. The washing fastness of dyed fabrics with organic salt was excellent. Experimental results showed that dye exhaustion reached the maximum value when the concentration range of organic salt was from 20 g/l to 40 g/l. In addition, the optimal alkali concentration in organic salt dyeing was found to be about 30 g/l. These parameters suggested organic salt could replace inorganic salt in reactive dyeing process.
Similar content being viewed by others
References
F. Zhang, Y. Chen, H. Lin, and Y. Lu, Color Technol., 123, 351 (2007).
N. Terinte, B. M. K. Manda, J. Taylor, K. C. Schuster, and M. K. Patel, J. Clean Prod., 72, 127 (2014).
K. Xie, F. Cheng, W. Zhao, and L. Xv, J. Clean Prod., 19, 332 (2011).
F. Ferrero, M. Periolatto, G. Rovero, and M. Giansetti, J. Clean Prod., 19, 1377 (2011).
J. M. Rosa, E. B. Tambourgi, and J. C. C. Santana, Text. Res. J., 84, 1009 (2014).
S. M. Burkinshaw, M. Mignanelli, P. E. Froehling, and M. J. Bide, Dyes Pigment., 47, 259 (2000).
J. P. Luttringer, Text. Chemist. Colorist, 5, 25 (1993).
K. Srikulkit and P. Santifuengkul, Color Technol., 116, 398 (2000).
M. Zhang, B. Ju, S. Zhang, W. Ma, and J. Z. Yang, Carbohydr. Polym., 69, 123 (2007).
L. Fang, X. Zhang, and D. Sun, Carbohydr. Polym., 91, 363 (2013).
M. Sadeghi-Kiakhani and S. Safapour, Fiber. Polym., 16, 1075 (2015).
M. Montazer, R. M. A. Malek, and A. Rahimi, Fiber. Polym., 8, 608 (2007).
H. G. Prabu and M. Sundrarajan, Color Technol., 118, 131 (2002).
Y. Guan, Q. Zheng, Y. Mao, M. Gui, and H. Fu, J. Appl. Polym. Sci., 105, 726 (2007).
L. Liu and J. Yao, AATCC Review, 11, 52 (2011).
A. Khatri, R. Padhyea, and M. White, Color Technol., 129, 76 (2013).
A. Khatri, Int. Proc. Econ. Dev. Res., 18, 84 (2011).
A. Khatri, M. H. Peerzada, M. Mohsin, and M. White, J. Clean Prod., 87, 50 (2015).
R. M. El-Shishtawy, Y. A. Youssef, N. S. E. Ahmed, and A. A. Mousa, Dyes Pigment., 72, 57 (2007).
N. S. E. Ahmed, Dyes Pigment., 65, 221 (2005).
D. Simijonovic, Z. D. Petrovic, and V. P. Petrovic, J. Mol. Liq., 179, 98 (2013).
L. Zhai, Q. Zhong, C. He, and J. Wang, J. Hazard. Mater., 177, 807 (2010).
D. Sun, Q. Guo, and X. Liu, Ultrasonics, 50, 441 (2010).
FCN. http://www.fda.gov/food/ingredientspackaginglabeling/ environmentaldecisions/ucm364268.htm.
FDA.http://www.fda.gov/downloads/food/ingredientspackaging labeling/environmentaldecisions/ucm143150.pdf.
M. I. Hossain, M. El-Harbawi, Y. A. Noaman, M. A. B Bustam, N. B. MAlitheen, N. A. Affandi, and C. Yin, Chemosphere, 84, 101 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, D., Zhang, X., Du, H. et al. Application of liquid organic salt to cotton dyeing process with reactive dyes. Fibers Polym 18, 1969–1974 (2017). https://doi.org/10.1007/s12221-017-1241-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-017-1241-3