Abstract
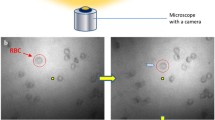
Cell biomechanical parameters, such as modulus of elasticity, are crucial biomarkers for indicating their physiological and pathological states. Traditionally, tethered techniques like atomic force microscopy (AFM), micropipette aspiration, and microinjection are used for the estimation of cell biomechanical parameters. These techniques being tethered are restricted by the direction of approach and require skilled manual interventions. This paper presents an untethered approach based on magnetic microrobot for estimating biomechanical parameters. We use spherical ferromagnetic particles as microrobots which when actuated by a global magnetic field produced by electromagnetic coils placed in quadrupole configuration, deform the target zebrafish embryos. The deformation is estimated using an image-based approach while the actuating magnetic force is determined using multiphysics simulations. We then employ a two-parameter Mooney-Rivlin model to estimate the biomechanical parameters. The developed approach is untethered and hence can be used for performing measurements from various directions, unlike the traditional tethered approaches. Furthermore, our approach can be used for studying large cells and their agglomerates without causing photodamage and oxidative stress that are associated with the conventional untethered approaches using optical and acoustic tweezers, respectively.







Similar content being viewed by others
Availability of data and materials
Available on reasonable request.
References
Shen Y, Nakajima M, Yang Z, Tajima H, Najdovski Z, Homma M, Fukuda T (2013) Single cell stiffness measurement at various humidity conditions by nanomanipulation of a nano-needle. Nanotechnology 24(14):145703
Pu H, Liu N, Yu J, Yang Y, Sun Y, Peng Y, Xie S, Luo J, Dong L, Chen H et al (2019) Micropipette aspiration of single cells for both mechanical and electrical characterization. IEEE Trans Biomed Eng 66(11):3185–3191
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3(4):413–438
Rianna C, Radmacher M (2016) Cell mechanics as a marker for diseases: Biomedical applications of AFM. AIP Conf Proc 1760(1):020057. https://pubs.aip.org/aip/acp/article-pdf/doi/10.1063/1.4960276/12828775/020057_1_online.pdf
Maciaszek JL, Lykotrafitis G (2011) Sickle cell trait human erythrocytes are significantly stiffer than normal. J Biomech 44(4):657–661
Zemła J, Bobrowska J, Kubiak A, Zieliński T, Pabijan J, Pogoda K, Bobrowski P, Lekka M (2020) Indenting soft samples (hydrogels and cells) with cantilevers possessing various shapes of probing tip. Eur Biophys J 49(6):485–495
Tan Y, Sun D, Huang W, Cheng SH (2008) Mechanical modeling of biological cells in microinjection. IEEE Trans Nanobioscience 7(4):257–266
Khangai N, Kojima M, Horade M, Kamiyama K, Sakai S, Mae Y, Arai T (2014) Cell stiffness measurement using two-fingered micro-hand equipped with plate-shaped end effector. In: 2014 11th international conference on ubiquitous robots and ambient intelligence (URAI), pp. 517–521. IEEE
Yousafzai MS, Ndoye F, Coceano G, Niemela J, Bonin S, Scoles G, Cojoc D (2016) Substrate-dependent cell elasticity measured by optical tweezers indentation. Opt Lasers Eng 76:27–33
Aermes C, Hayn A, Fischer T, Mierke CT (2020) Environmentally controlled magnetic nano-tweezer for living cells and extracellular matrices. Sci Rep 10(1):1–16
Lim HG, Liu H-C, Yoon CW, Jung H, Kim MG, Yoon C, Kim HH, Shung KK (2020) Investigation of cell mechanics using single-beam acoustic tweezers as a versatile tool for the diagnosis and treatment of highly invasive breast cancer cell lines: an in vitro study. Microsyst Nanoeng 6(1):1–12
Luo Y, Chen D, Zhao Y, Wei C, Zhao X, Yue W, Long R, Wang J, Chen J (2014) A constriction channel based microfluidic system enabling continuous characterization of cellular instantaneous young’s modulus. Sens. Actuators B Chem 202:1183–1189
Adam G, Hakim M, Solorio L, Cappelleri DJ (2020) Stiffness characterization and micromanipulation for biomedical applications using the vision-based force-sensing magnetic mobile microrobot. In: 2020 international conference on manipulation, automation and robotics at small scales (MARSS), pp. 1–6. IEEE
Zhang H, Liu K-K (2008) Optical tweezers for single cells. J R Soc Interface 5(24):671–690
Wang X, Ho C, Tsatskis Y, Law J, Zhang Z, Zhu M, Dai C, Wang F, Tan M, Hopyan S et al (2019) Intracellular manipulation and measurement with multipole magnetic tweezers. Sci Robot 4(28):6180
Li M, Dang D, Liu L, Xi N, Wang Y (2017) Atomic force microscopy in characterizing cell mechanics for biomedical applications: A review. IEEE Trans Nanobioscience 16(6):523–540
Wang X, Law J, Luo M, Gong Z, Yu J, Tang W, Zhang Z, Mei X, Huang Z, You L et al (2020) Magnetic measurement and stimulation of cellular and intracellular structures. ACS Nano 14(4):3805–3821
Zeng D, Juzkiw T, Read AT, Chan DW-H, Glucksberg MR, Ethier CR, Johnson M (2010) Young’s modulus of elasticity of schlemm’s canal endothelial cells. Biomech Model Mechanobiol 9(1):19–33
Bausch AR, Möller W, Sackmann E (1999) Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76(1):573–579
Kawahara T, Sugita M, Hagiwara M, Arai F, Kawano H, Shihira-Ishikawa I, Miyawaki A (2013) On-chip microrobot for investigating the response of aquatic microorganisms to mechanical stimulation. Lab Chip 13(6):1070–1078
Hochmuth RM (2000) Micropipette aspiration of living cells. J Biomech 33(1):15–22. https://doi.org/10.1016/S0021-9290(99)00175-X
Pors S, Nikiforov D, Cadenas J, Ghezelayagh Z, Wakimoto Y, Jara L, Cheng J, Dueholm M, Macklon K, Flachs E et al (2022) Oocyte diameter predicts the maturation rate of human immature oocytes collected ex vivo. J Assist Reprod Genet 39(10):2209–2214
Chen J, Niu N, Zhang J, Qi L, Shen W, Donkena KV, Feng Z, Liu J (2019) Polyploid giant cancer cells (pgccs): the evil roots of cancer. Curr Cancer Drug Targets 19(5):360–367
Tang X, Liu X, Li P, Liu D, Kojima M, Huang Q, Arai T (2021) Efficient single-cell mechanical measurement by integrating a cell arraying microfluidic device with magnetic tweezer. IEEE Robot Autom Lett 6(2):2978–2984
Nakajima M, Ahmad MR, Kojima S, Homma M, Fukuda T (2009) Local stiffness measurements of c. elegans by buckling nanoprobes inside an environmental sem. In: 2009 IEEE/RSJ international conference on intelligent robots and systems, pp. 4695–4700. IEEE
Wang X, Zhang X (2019) Biomechanical study on elastic and viscoelastic properties of osteoblasts using atomic force microscopy. In: 2019 IEEE international conference on mechatronics and automation (ICMA), pp. 1377–1381. https://doi.org/10.1109/ICMA.2019.8816579
Nam JH, Chen PC, Lu Z, Luo H, Ge R, Lin W (2010) Mechanoinduction of reduction in the stiffness of zebrafish chorion. In: 2010 11th international conference on control automation robotics & Vision, pp. 1024–1028. IEEE
Kim D-H, Hwang CN, Sun Y, Lee SH, Kim B, Nelson BJ (2006) Mechanical analysis of chorion softening in prehatching stages of zebrafish embryos. IEEE Trans Nanobioscience 5(2):89–94
Liu F, Wu D, Chen K (2014) A zebrafish embryo behaves both as a “cortical shell- liquid core” structure and a homogeneous solid when experiencing mechanical forces. Microsc Microanal 20(6):1841–1847
Schoetz E-M (2007) Dynamics and mechanics of zebrafish embryonic tissues. PhD thesis, Dresden, Techn. Univ., Diss., 2007
Agarwal D, Thakur AD, Thakur A (2022) Magnetic microbot-based micromanipulation of surrogate biological objects in fluidic channels. J Microbio Robot 1–15
Lee H, Lee D, Jeon S (2022) A two-dimensional manipulation method for a magnetic microrobot with a large region of interest using a triad of electromagnetic coils. Micromachines 13(3). https://doi.org/10.3390/mi13030416
Agarwal D, Thakur AD, Thakur A (2019) A robotic tool for magnetic micromanipulation of cells in the presence of an ambient fluid flow. In: International design engineering technical conferences and computers and information in engineering conference, vol. 59223, pp. 004–08017. American Society of Mechanical Engineers
Agarwal D, Thakur AD, Thakur A (2022) A feedback-based manoeuvre planner for nonprehensile magnetic micromanipulation of large microscopic biological objects. Robot Auton Syst 148:103941
Amokrane W, Belharet K, Souissi M, Grayeli AB, Ferreira A (2018) Macro-micromanipulation platform for inner ear drug delivery. Robot Auton Syst 107:10–19
Yesin KB, Vollmers K, Nelson BJ (2006) Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int J Rob Res 25(5–6):527–536
Yasukuni R, Minamino D, Iino T, Araki T, Takao K, Yamada S, Bessho Y, Matsui T, Hosokawa Y (2021) Pulsed laser activated impulse response encoder (plaire): sensitive evaluation of surface cellular stiffness on zebrafish embryos. Biomed Opt Express 12(3):1366–1374
Tomizawa Y, Dixit K, Daggett D, Hoshino K (2019) Biocompatible cantilevers for mechanical characterization of zebrafish embryos using image analysis. Sensors 19(7):1506
Wu P-H, Aroush DR-B, Asnacios A, Chen W-C, Dokukin ME, Doss BL, Durand-Smet P, Ekpenyong A, Guck J, Guz NV et al (2018) A comparison of methods to assess cell mechanical properties. Nat Methods 15:491–498
Kontomaris SV, Stylianou A, Georgakopoulos A, Malamou A (2023) Is it mathematically correct to fit afm data (obtained on biological materials) to equations arising from hertzian mechanics? Micron 164:103384. https://doi.org/10.1016/j.micron.2022.103384
Rawson D, Zhang T, Kalicharan D, Jongebloed W (2000) Field emission scanning electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish brachydanio rerio. Aquac Res 31:325–336. https://doi.org/10.1046/j.1365-2109.2000.00401.x
Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ (2010) Octomag: An electromagnetic system for 5-dof wireless micromanipulation. IEEE Trans Robot 26(6):1006–1017. https://doi.org/10.1109/TRO.2010.2073030
Zhou H, Mayorga-Martinez CC, Pané S, Zhang L, Pumera M (2021) Magnetically driven micro and nanorobots. Chem Rev 121(8):4999–5041
Beicker K, O’Brien E, Falvo M, Superfine R (2018) Vertical light sheet enhanced side-view imaging for afm cell mechanics studies. Sci Rep 8. https://doi.org/10.1038/s41598-018-19791-3
Ren Y, Keshavarz M, Anastasova S, Hatami G, Lo B, Zhang D (2022) Machine learning-based real-time localization and automatic trapping of multiple microrobots in optical tweezer. In: 2022 international conference on manipulation, automation and robotics at small scales (MARSS), pp. 1–6. https://doi.org/10.1109/MARSS55884.2022.9870467
Cui G, Zhang P, Liu X, Xie L, Huang W, Pan P, Qu J, Fan Q (2023) Novel coil array design and modeling for independent control of multiple magnetic microrobots. IEEE Trans Ind Electron 70(10):10302–10311. https://doi.org/10.1109/TIE.2022.3222626
Chowdhury S, Jing W, Cappelleri DJ (2015) Controlling multiple microrobots: recent progress and future challenges. J Microbio Robot 10(1):1–11
Acknowledgements
This work was supported by the Indian Institute of Technology Patna, Patna, Bihar, India. We express our sincere gratitude to Dr. Deepak Kumar Sinha, Associate Professor, School of Biological Sciences, Indian Association for the Cultivation of Science, Kolkata, India, for his assistance with zebrafish embryos.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.T., A.D.T., and A.R. Investigation, Formal analysis, and Writing: D.A., and Y.K. contributed equally. Supervision and Draft Revision: A.T.
Corresponding author
Ethics declarations
Conflict of interest/Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 3 (mp4 1272 KB)
Index
Index
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agarwal, D., Kamble, Y., Raj, A. et al. Biomechanical parameter estimation using untethered nonprehensile magnetic microrobot. J Micro-Bio Robot 19, 59–70 (2023). https://doi.org/10.1007/s12213-023-00164-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12213-023-00164-7