Abstract
The nodal explants of C. ovalifolium were cultured on Murashige and Skoog (MS) medium augmented with either [6-benzyladenine (BA) or kinetin (Kn) or thidiazuron (TDZ) or N6-(2-isopentenyl) adenine (2 ip)]. The maximum percentage response (100%) with highest number of shoots (5.0 ± 0.9) with longest shoot (3.7 ± 0.5 cm) per nodal explants were obtained on MS medium containing 5 mg/l BA after 45 days of culture. Shoot regenerated on MS medium supplemented with 5 mg/l BA as well as from other cytokinin shows callus formation at the base of stem; hence these shoots are not suitable for further use. The possible reason of callus formation could be ascribed to the activity of endogenous auxin. To prevent callus formation, nodal explants were cultured on MS medium supplemented with combination of BA and 2,3,5-tri-iodobenzoic acid (TIBA) or BA with TIBA and activated charcoal or BA with TIBA and adenine sulfate (AdS). Nodal explants inoculated on the MS medium augmented with 5 mg/l BA, 3 mg/l TIBA and 30 mg/l AdS have not only prevented callus formation but also produced significantly maximum number of shoots (7.5 ± 0.3) having shoot length of 4.7 ± 0.2. Maximum number of roots (6.3 ± 0.4) and root length (5.3 ± 0.1 cm) per explant was observed on half-strength MS medium containing 2 mg/l indole 3-butyric acid.
Similar content being viewed by others
1 Introduction
The Combretaceae is a large family of herbs, shrubs and trees, consisting of nearly 20 genera and 600 species having tropical distribution around the world and centers of diversity in Asia and Africa, and most of them are used for medicinal purpose traditionally (Fyhrquist et al. 2002; de Morais Lima et al. 2012). Combretum species are widely utilized in traditional medicine for diabetes, infections, inflammation, malaria, bleeding, digestive and diarrheal problems (de Morais ima et al. 2012). Combretum ovalifolium Roxb. is a deciduous climber, leaves, elliptic-ovate, apex obtusely accuminate, base cuneate, coriaceous, spikes 1–3 cm, petals oblong, fruits 2–2.5 cm long, obovate, coriaceous, golden yellow with 4-papery wings, four-winged thin seeds (Roy et al. 1992). The presence of fibers with protoplast is a characteristic feature of C. ovalifolium. Prismatic crystals frequently co-occur with the nucleus in the same compartment of the fibers (Rajput and Rao 1999). Seed oil contains 35.4% of palmitic acid, 16.4% myristic acid, 24% of oleic, 17.1% linoleic acids and a small quantity of lauric, stearic, arachidic and behenic acids (Daulatabad and Ankalgi 1983). Leaves of C. ovalifolium are known to have mosquito repellent (Singh and Saratchandra 2005; Modi and Mathad 2016) and insect repellent activity (Singh and Saratchandra 2005) and traditionally utilized for control of insect pest by local farmers (Gupta and Patel 1992). Extracts of C. ovalifolium show larvicidal properties against vector Anopheles stephensi (Krishnappa et al. 2013). Mahida and Mohan (2006) reported the antibacterial activity of C. ovalifolium. Stem bark juice of C. ovalifolium is administered orally as a cure for jaundice (Sanjeev et al. 1997; Vaidyanathan et al. 2013; Nandagoapalan et al. 2016). A decoction of the bark of Albezia procera with the bark of C. ovalifolium and Acacia ferruginea and roots of Blumea eriantha is administered as an antidote for snakebite (Quattrocchi 2012). C. ovalifolium is used as a source of fiber-yielding plant in South Gujarat, India (Gavali and Sharma 2004). A decoction of leaves is used for curing of menstrual problems in Amravati, Maharashtra (Onkar 2016). This is a fiber-yielding plant (Gavali and Sharma 2004) and, therefore, utilized for rope making (Rao et al. 2015). Terminal panicle of C. ovalifolium is crushed and used as local antiseptic and antibiotic (Panda 2002). Combretum sp propagates by seed germination (Onyekwelu 1990; Dalling and Van Staden 1999; Wickens and Gaum 2001). Information about seed germination or any vegetative method of propagation, for C. ovalifolium, is not available. Conventional breeding methods are only limited to the agriculturally important plants, especially plants having a short life cycle. Therefore, such techniques cannot be used for slow growing, long-lived, highly heterozygous and sexually self-incompatible plants (Hammatt 1992). Micropropagation techniques are the popular choice for propagation and conservation of medicinal plants (Nataraj et al. 2016; Kher et al. 2016a; Teixeira da Silva et al. 2016). There have been many studies on the micropropagation of Combretaceae members using nodal explants like Anogeissus sericea var. nummularia King ex. Duthie (Yusuf 2005), Terminalia bellirica Roxb. (Ramesh et al. 2005; Phulwaria et al. 2012a), T. arjuna Roxb. (Pandey et al. 2006), T. catappa Linn (Phulwaria et al. 2012b). The objective of the present research work was propagation of C. ovalifolium through axillary shoot multiplication and in vitro rooting of regenerated plants.
2 Materials and methods
2.1 Plant material and surface sterilization
Nodal explants were collected from Medicinal Plant Garden, Anand Agriculture University, Gujarat, India (Fig. 1a). Nodal explants were carefully washed under running tap water for 5 min and then soaked in 3% Neutral Rankleen detergent (v/v) (RFCL-India) for 10 min followed by 10 min washing in running tap water. Subsequently, the explants were treated with 0.1% mercuric chloride (w/v) solution for 3 min and then rinsed 3–4 times with sterile distilled water (DW).
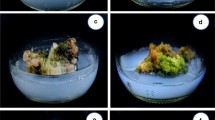
Axillary shoot multiplication of C. ovalifolium. a C. ovalifolium, b M.S. medium supplemented with 5 mg/l BA + 3 mg/l TIBA + 2 gm/l activated charcoal, c, d callus formation from nodal explant, e MS basal medium, f MS + 5 mg/l TDZ, g MS + 5 mg/l mg/l BA. (Arrow indicates vitrified shoots, circle indicates callus formation)
2.2 Composition of culture media and culture conditions for in vitro cultures
The culture medium used was Murashige and Skoog (1962): MS medium salts, vitamins with 3% (w/v) sucrose. The pH of all media was adjusted to 5.8 with 0.1 N NaOH or HCl before adding 0.8% (w/v) agar (bacteriological grade; Merck) and autoclaved at 121 °C for 15 min. Cultures were maintained at 25 ± 2 °C under a 16-h photoperiod at a photosynthetic photon flux density of 35 µmol m−2 s−1 provided by cool white fluorescent tubes (36 W Philips).
2.3 Effects of cytokinin on axillary shoot multiplication
Surface sterilized nodal explants of 1.5–2.0 cm height with one node (with two axillary meristems) were inoculated with MS medium containing 1.0–5.0 mg/l 6-benzyaminopurine (BA) or kinetin (Kn) or 2-isopentenyladenine (2iP) or thidiazuron (TDZ). Within 15 days of culture, prolific callus formation (Fig. 1c, d) was observed at the cut ends of nodal explants, which inhibited multiplication of shoot (Table 1).
2.4 Effects of additives on axillary shoot multiplication
For prevention of callus formation, nodal explants were cultured on MS medium augmented with combination of 5 mg/l BA and 1, 3 and 5 mg/l of 2,3,5-tri-iodobenzoic acid (TIBA) or 5 mg/l BA with 3 mg/l TIBA and 1, 2 and 3 g/l activated charcoal (AC) or 5 mg/l BA with 3 mg/l TIBA and 15, 30, 45 and 60 mg/l adenine sulfate (AdS) Table 4.
2.5 Rooting and acclimatization
In vitro raised shoots of C. ovalifolium were rooted on half-strength MS medium supplemented with various concentrations of auxins (Table 6). Regenerated plantlets of C. ovalifolium with well-developed roots were removed from rooting medium and washed gently under running tap water to remove medium adhering to roots. Subsequently, plantlets were transferred to plastic cups containing cocopeat, soil mixture and sterile sand in 1:1:1 ratio. The transplanted plants were covered with clear transparent plastic bags to retain humidity. Pots containing micropropagated plants of C. ovalifolium were kept in plant tissue culture laboratory and watered with 15 ml of quarter-strength MS medium (quarter-strength of macro- and micronutrients) at 4-day intervals for 15 days. After 15 days, the plastic cover was removed and the uncovered plants were maintained at 25 °C under a 16-h photoperiod and 35 µmol m−2 s−1 in the laboratory for 30 days. After 30 days, plantlets were transferred to the field under natural conditions.
2.6 Statistical analysis
All experiments were conducted with 24 replicates (i.e., explants) per treatment, evaluated after 45 days and evaluated after 45 days. Data were subjected to analysis of variance (ANOVA) to assess treatment differences and interaction, and Duncan’s Multiple Range Test using SPSS-19 for (SPSS Inc., Chicago, IL, USA) calculated the significance of differences.
3 Results and discussion
3.1 Effects of cytokinins on axillary shoot multiplication
Axillary shoot multiplication is the popular method of micropropagation due to the presence of predetermined meristem for shoot formation (Saha et al. 2016; Shekhawat and Manokari 2016). For axillary shoot multiplication, type and concentration of cytkinin play significant roles. Therefore, in this study the response of nodal explants to various concentrations of BA, Kn, TDZ and 2-ip is investigated (Tables 1, 2 and 3). The response of multiple shoot induction varied significantly on MS basal medium with respect to different concentrations of cytokinin type and concentration. Nodal explants exhibited bud breaking within 15 days (Table 1). 66% of nodal explants cultured on basal MS medium exhibited shoot regeneration without any callus formation. 100% regeneration with shoot per node was observed from explants cultured on MS medium augmented with 5 mg/l BA. It was observed that excessive callus formation from the base of a node in all media augmented with cytokinins (Fig. 1c, d, e). Higher concentration (2, 3, 4 and 5 mg/l) of TDZ caused callusing and vitrification of shoots (Fig. 1f). Formation of callus hinders the quality of shoots which are not suitable for further subculture and rooting. Callus formation from nodal explants in cytokinin-enriched MS medium is maybe due to the presence of high concentration of endogenous auxin, which leads to wound healing (Bernabé-Antonio et al. 2012; Ikeuchi et al. 2013). These results indicate that a very high level of BA is necessary for maximal shoot proliferation. Vitrification of shoots due to TDZ has been reported in Pluchea lanceolata (Kher et al. 2014), Aloe polyphylla (Ivanova and Van Staden 2010) and Citrullus lanatus (Vinoth and Ravindhran 2015).
3.2 Effects of additives on axillary shoot multiplication
Anti-auxin 2,3,5 tri-iodobenzoic acid (TIBA), a well-known polar auxin transport inhibitor, adenines sulfate (AdS) and activated charcoal were used to study effects on shoot multiplication from nodal explants of C. ovalifolium (Table 4). 100% axillary shoot multiplication was observed in all concentrations and combinations of plant growth regulators (Table 4). Excellent shoot formation was observed within 30 days on MS medium augmented with 5 mg/l BA, 3 mg/l TIBA and 30 mg/l AdS (Fig. 2b). Best results were observed on the same medium (7.5 shoots/node with average 4.7 cm shoot length); it was noted that when cultures are kept for 45 days, it showed root formation from nodal regions (Fig. 2b). Application of AdS and AC enhances shoot multiplication and shoot length (Fig. 1b; Tables 4, 5). The Increase in concentration of AdS from 15 mg/l to 30 mg/l increased shoot multiplication and shoot length while beyond 30 mg/l did not show any further increase in shoot number and length. Application of AdS for axillary shoot multiplication of other Combretaceae plants are reported for, e.g., 50 mg/l for Terminalia arjuna (Choudhary et al. 2015), 25 mg/l for Terminalia catappa (Phulwaria et al. 2012b) and Anogeissus latifolia (Shekhawat et al. 2000). TIBA controls the polar transport of auxin from cell to cell by adjusting the efflux carrier for auxin (Li et al. 2012). Bhau and Wakhlu (2001) reported that TIBA decreases the accumulation of auxin by suppressing the transport of endogenous auxins in the callus cells. As in the present study, Lall et al. (2005) found that TIBA promoted axillary shoot multiplication and reduced callus formation in Alnus glutinosa. The inclusion of TIBA in the culture media promoted axillary bud break and reduced callus in Capsicum sp (Christopher and Rajam 1994), Alnus glutinosa (Lall et al. 2005), Cucumis sativus (Shukla et al. 2014) and Clerodendrum phlomidis (Kher et al. 2016b).
Axillary shoot multiplication of C. ovalifolium. a MS + 5 mg/l BA + 3 mg/l TIBA + 30 mg/l AdS photographed after 30 days, b MS + 5 mg/l BA + 3 mg/l TIBA + 30 mg/l AdS photographed after 45 days, c ½ MS + 2 mg/l IBA, d PGR free ½ MS medium, e Acclimatized micropropagated plant after 60 days in natural condition
3.3 Rooting and acclimatization
Rooting and acclimatization of in vitro raised shoots is a crucial step of micropropagation of any plants (Hazarika 2003; Osório et al. 2013). For root induction, individual microshoots were transferred to half-strength MS medium augmented with different concentrations of auxins (IAA, NAA and IBA). In vitro rooting (100%) was observed in MS medium augmented with auxins as well as on auxin free half-strength MS medium (Table 6; Fig. 2d). Two-way ANOVA of auxin type and concentration had no significant effect on number of root per shoot (Table 7) and root length (Table 8). Formation of roots on auxin-free medium is a possible indication of the presence of endogenous auxin. Best results were obtained on half-strength MS medium augmented with 2 mg/l IBA (6.3 roots/shoot with average root length of 5.3 cm). The effectiveness of IBA over other auxin for in vitro rooting is reported for other Combretaceae members such as Quisqualis indica (Mandal 2013). In the present research work, 85% of plants successfully survived (Fig. 2e) under natural conditions.
4 Conclusion
In conclusion, the present work describes a regeneration method for C. ovalifolium from the nodal explants. Micropropagation of C. ovalifolium was achieved by supplementing MS medium with 3 mg/l TIBA, an inhibitor of auxin transport plus 5 mg/l BA with 30 mg/l AdS for shoot multiplication, and 2 mg/l IBA for rooting of regenerated shoots. This protocol could be viable option for large-scale propagation of other Combretum species.
References
Bernabé-Antonio A, Santacruz-Ruvalcaba F, Cruz-Sosa F (2012) Effect of plant growth regulators on plant regeneration of Dioscorea remotiflora (Kunth) through nodal explants. Plant Growth Regul 68:293–301. doi:10.1007/s10725-012-9717-z
Bhau BS, Wakhlu AK (2001) Effect of genotype, explant type and growth regulators on organogenesis in Morus alba. Plant Cell Tissue Organ Cult 66:25–29. doi:10.1023/A:1010617212237
Choudhary M, Jaiswal S, Singh R, Dev I, Sarita A (2015) A micropropagation protocol for mass multiplication of Terminalia arjuna-a valuable medicinal tree. Adv For Sci 2:1–6
Christopher T, Rajam MV (1994) In vitro clonal propagation of Capsicum spp. Plant Cell Tissue Organ Cult 38:25–29. doi:10.1007/BF00034439
Dalling KJ, Van Staden J (1999) Germination requirements of Combretum bracteosum seeds. South Afr J Bot 65:83–85. doi:10.1016/S0254-6299(15)30943-1
Daulatabad CD, Ankalgi RE (1983) Component fatty acids of some Indian seed oils. Fette Seifen Anstrichm 85:404–406. doi:10.1002/lipi.19830851007
de Morais Lima GR, de Sales IRP, Caldas Filho MRD, de Jesus NZT, de Sousa Falcão H, Barbosa-filho JM, Cabral AGS, Souto AL, Tavares JF, Batista LM, Lima GRD, Caldas MRD, Falcao HD, Barbosa JM, Rodrigues G, Lima DM, Rafael I, De Sales P, Ricardo M, Caldas D, Zínia N, De Jesus T, Falcão HDS, Guedes A, Cabral S (2012) Bioactivities of the Genus Combretum (Combretaceae): a review. Molecules 17:9142–9206. doi:10.3390/molecules17089142
Fyhrquist P, Mwasumbi L, Hæggström C-A, Vuorela H, Hiltunen R, Vuorela P, Haeggström C-A, Vuorela H, Hiltunen R, Vuorela P (2002) Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J Ethnopharmacol 79:169–177. doi:10.1016/S0378-8741(01)00375-0
Gavali D, Sharma D (2004) Traditional knowledge and biodiversity conservation in Gujarat. Indian J Tradit Knowl 3:51–58
Gupta AK, Patel KK (1992) Survey of farmers’ innovations in Gujarat: part-III-insect pest control. Honey Bee 3:17–19
Hammatt N (1992) Progress in the biotechnology of trees. World J Microbiol Biotechnol 8:369–377. doi:10.1007/BF01198747
Hazarika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85:1704–1712
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25:3159–3173. doi:10.1105/tpc.113.116053
Ivanova M, Van Staden J (2010) Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tissue Organ Cult 104:13–21. doi:10.1007/s11240-010-9794-5
Kher MM, Joshi D, Nekkala S, Nataraj M, Raykundaliya DP (2014) Micropropagation of Pluchea lanceolata (Oliver & Hiern.) using nodal explant. J Hort Res 22:35–39. doi:10.2478/johr-2014-0004
Kher MM, Nataraj M, Teixeira da Silva JA (2016a) Micropropagation of Crataeva L. species. Rend Fis Acc Lincei 27:157–167. doi:10.1007/s12210-015-0478-2
Kher MM, Soner D, Srivastava N, Nataraj M, Teixeira da Silva JA (2016b) Micropropagation of Clerodendrum phlomidis L.F. J Hort Res 24:21–28. doi:10.1515/johr-2016-0003
Krishnappa K, Mathivanan T, Elumalai A, Jeyasankar A, Dhanasekaran S, Elumalai K (2013) Evaluation of Cissus quadrangularis and Combretum ovalifolium medicinal plants extracts against medically important human malarial vector mosquito Anopheles stephensi Liston (Diptera: Culicidae). Int J Interdiscip Res Rev 1:11–18
Lall S, Mandegaran Z, Roberts AV (2005) Shoot multiplication in cultures of mature Alnus glutinosa. Plant Cell Tissue Organ Cult 83:347–350. doi:10.1007/s11240-005-7014-5
Li YH, Zou MH, Feng BH, Huang X, Zhang Z, Sun GM (2012) Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol Biochem 55:33–42. doi:10.1016/j.plaphy.2012.03.012
Mahida Y, Mohan JSS (2006) Screening of Indian plant extracts for antibacterial activity. Pharm Biol 44:627–631. doi:10.1080/13880200600897551
Mandal J (2013) In vitro regeneration of Rangoon creeper (Quisqualis indica Linn.). Indian J Biotechnol 12:415–419
Modi RK, Mathad P (2016) Enumeration of angiosperm medicinal plants of Gavisiddalingeshwara sacred grove, Chintanpalli of Yadgir District, Karnataka. J Glob Biosci 5:3539–3558
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nandagoapalan V, Doss A, Marimuthu C (2016) Ethanobotanical studies on useful plants of Pachamalai hills of Tiruchirappalli district of Tamilnadu. J Adv Sci Res 7:14–19
Nataraj M, Kher MM, Teixeira da Silva JA (2016) Micropropagation of Clerodendrum L. species: a review. Rend Fis Acc Lincei 27:169–179. doi:10.1007/s12210-015-0484-4
Onkar AA (2016) Ethanobotanical notes from Pohara-Malkhed reserve forest, Amravati, Maharastra, India. Bio Bull 2:107–111
Onyekwelu SSC (1990) Germination, seedling morphology and establishment of Combretum bauchiense Hutch. & Dalz. (Combretaceae). Bot J Linn Soc 103:133–138. doi:10.1111/j.1095-8339.1990.tb00179.x
Osório ML, Gonçalves S, Coelho N, Romano A, Osório J (2013) How to monitor the acclimatization of micropropagated plants-from in vitro to the field? Acta Hortic 988:65–70
Panda H (2002) Combretum ovalifolium. In: Medicinal Plant Cultivation and their uses. Asian Pacific Buisness Press Inc., Kamla Nagar, Delhi, India, p 148
Pandey S, Singh M, Jaiswal U, Jaiswal VS (2006) Shoot initiation and multiplication from a mature tree of Terminalia arjuna Roxb. In Vitro Cell Dev Biol Plant 42:389–393. doi:10.1079/IVP2006790
Phulwaria M, Rai MK, Gupta AK, Ram K, Shekhawat NS (2012a) An improved micropropagation of Terminalia bellirica from nodal explants of mature tree. Acta Physiol Plant 34:299–305. doi:10.1007/s11738-011-0828-3
Phulwaria M, Ram K, Harish Gupta AK, Shekhawat NS (2012b) Micropropagation of mature Terminalia catappa (Indian Almond), a medicinally important forest tree. J For Res 17:202–207. doi:10.1007/s10310-011-0295-0
Quattrocchi U (2012) World dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology. CRC Press, London (5 volume set)
Rajput KS, Rao KS (1999) Nucleated wood fibres in some members of Combretaceae. IAWA J 20:79–83
Ramesh M, Umate P, Venugopal Rao K, Sadanandam A (2005) Micropropagation of Terminalia bellirica Roxb.—a sericulture and medicinal plant. In Vitro Cell Dev Biol Plant 41:320–323. doi:10.1079/IVP2004626
Rao DS, Rao VS, Murthy PP, Roa GMN, Rao YV (2015) Some ethno medicinal plants of Parnasala sacred grove area Eastern Ghats of Khammam District, Telangana, India. J Pharm Sci Res 7:210–218
Roy GP, Shukla BK, Datt B (1992) Combretum ovalifolium Roxb. In: Flora of Madhya Pradesh (Chattarpur and Damoh). Aashish Publishing House, 8/81Panjabi Bagh, New Delhi, India, p 172
Saha S, Dey T, Adhikari S, Mukhopadhyay S, Sengupta C, Ghosh P (2016) Effects of plant growth regulators on efficient plant regeneration efficiency and genetic stability analysis from two Ocimum tenuiflorum L. morphotypes. Rend Fis Acc Lincei 27:609-628. doi:10.1007/s12210-016-0538-2
Sanjeev KK, Sasidharan N, Sajeev KK (1997) Ethanobotanical observations on tribals of Chinnar wildlife sanctuary. Anc Sci Life 16:284–292
Shekhawat MS, Manokari M (2016) In vitro regeneration frequency, micro-morphological studies and ex vitro rooting of Hemidesmus indicus (L.) R. Br.: a multi-potent endangered climber. Indian J Plant Physiol 21:151–160. doi:10.1007/s40502-016-0216-5
Shekhawat NS, Yadav J, Arya V, Singh RP (2000) Micropropagation of Anogeissus latifolia (Roxb. Ex DC.) Wall, ex Guill. & Perr.-a tree of fragile ecosystems. J Sustain For 11:83–96. doi:10.1300/J091v11n04_05
Shukla PS, Das AK, Jha B, Agarwal PK (2014) High-frequency in vitro shoot regeneration in Cucumis sativus by inhibition of endogenous auxin. In Vitro Cell Dev Biol Plant 50:729–737. doi:10.1007/s11627-014-9649-6
Singh RN, Saratchandra B (2005) The development of botanical products with special reference to seri-ecosystem. Casp J Environ Sci 3:1–8
Teixeira da Silva JA, Kher MM, Nataraj M (2016) Biotechnological advances in Vitex species, and future perspectives. J Genet Eng Biotechnol 14:335–348. doi:10.1016/j.jgeb.2016.09.004
Vaidyanathan D, Senthilkumar MSS, Basha MG (2013) Studies on ethnomedicinal plants used by Malayali tribals in Kolli hills of Eastern Ghats, Tamilnadu, India. Asian J Plant Sci Res 3:29–45
Vinoth A, Ravindhran R (2015) Reduced hyperhydricity in watermelon shoot cultures using silver ions. In Vitro Cell Dev Biol Plant 51:258–264. doi:10.1007/s11627-015-9698-5
Wickens P, Gaum WG (2001) Scarification and stratification of Combretum erythrophyllum (Burchell) seed and fruit for uniformity in germination. South Afr J Plant Soil 18:171–173. doi:10.1080/02571862.2001.10634425
Yusuf A (2005) Clonal propagation of Anogeissus sericea var. nummularia- a rare tree of arid forestry. J Sustain For 20:67–78. doi:10.1300/J091v20n01_05
Acknowledgements
We are thankful Dr. Vinay R. Patel for photography assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Kher, M.M., Nataraj, M. Micropropagation of Combretum ovalifolium Roxb.: a medicinally important plant. Rend. Fis. Acc. Lincei 28, 519–527 (2017). https://doi.org/10.1007/s12210-017-0625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-017-0625-z