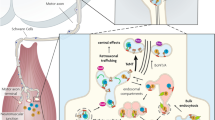
Abstract
Tetanus and botulinum neurotoxins, produced by anaerobic bacteria of the genus Clostridium, are the most toxic proteins known and are solely responsible for the pathogenesis of tetanus and botulism. They are metallo-proteases that enter nerve terminals and cleave proteins of the neuroexocytosis apparatus causing a persistent, but reversible, inhibition of neurotransmitter release. Botulinum neurotoxins are used in the therapy of many human syndromes caused by hyperactive nerve terminals. Snake presynaptic PLA2 neurotoxins block nerve terminals by binding to the nerve membrane and catalyzing phospholipid hydrolysis with production of lysophospholipids and fatty acids. These compounds change the membrane conformation causing enhanced fusion of synaptic vesicle via hemifusion intermediate with release of neurotransmitter and, at the same time, inhibition of vesicle fission and recycling. It is possible to envisage clinical applications of the lysophospholipid/fatty acid mixture to inhibit hyperactive superficial nerve terminals.
Similar content being viewed by others
Abbreviations
- ACh:
-
acetylcholine
- BoNT:
-
botulinum neurotoxin
- FA:
-
fatty acid
- LPL:
-
lysophospholipid
- NMJ:
-
neuromuscular junction
- SNAP-25:
-
25-kDa synaptosomal-associated protein
- SNARE:
-
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP:
-
vesicle-associated membrane protein
- SPAN:
-
snake presynaptic PLA2 neurotoxin
- SV:
-
synaptic vesicles
- TeNT:
-
tetanus neurotoxin
References
Abe T, Limbrick AR, Miledi R (1976) Acute muscle denervation induced by betabungarotoxin. Proc R Soc Lond B Biol Sci. 194: 545–553
Aoki KR (2001) A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon 39: 1815–1820
Bleck TP (1989) Clinical aspects of tetanus. In: Simpson LL (ed.), Botulinum neurotoxins and tetanus toxin. San Diego: Academic Press: 379–398
Bonanomi D, Pennuto M, Rigoni M, Rossetto O, Montecucco C, Valtorta F (2005) Taipoxin induces synaptic vesicle exocytosis and disrupts the interaction of synaptophysin I with VAMP2. Mol. Pharmacol. 67: 1901–1908
Chen IL, Lee CY (1970) Ultrastructural changes in the motor nerve terminals caused by beta-bungarotoxin. Virchows Arch. B. Cell Pathol. 6: 318–325
Chernomordik LV, Leikina E, Frolov V, Bronk P, Zimmerberg J (1997) An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell. Biol. 136: 81–93
Chernomordik LV, Kozlov MM (2003) Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72: 175
Connolly S, Trevett AJ, Nwokolo NC, Lalloo DG, Naraqi S, Mantle D, Schofield IS, Fawcett PR, Harris JB, Warrell DA (1995) Neuromuscular effects of Papuan Taipan snake venom. Ann. Neurol. 38: 916–920
Criado M, Gil A, Viniegra S, Gutierrez LM (1999) A single amino acid near the C terminus of the synaptosome-associated protein of 25 kDa (SNAP-25) is essential for exocytosis in chromaffin cells. Proc. Natl. Acad. Sci. USA 96: 7256–7261
Cull-Candy SG, Fohlman J, Gustavsson D, Lullmann-Rauch R, Thesleff S (1976) The effects of taipoxin and notexin on the function and fine structure of themurine neuromuscular junction. Neuroscience 1: 175–180
Dixon RW, Harris JB (1999) Nerve terminal damage by beta-bungarotoxin: its clinical significance. Am J Pathol. 154: 447–455
Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS (1997) Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J. Biol. Chem. 272: 21488–21494
Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER (2003) Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell. Biol. 162: 1293–1303
Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER (2006) SV2 Is the Protein Receptor for Botulinum Neurotoxin A. Science 312: 592–596
Duchen LW (1971) An electron microscopic study of the changes induced by botulinum toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 14: 47–60
Fischer A, Montal M (2007a) Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc.Natl.Acad. Sci.USA 104: 10447–10452
Fischer A, Montal M (2007b) Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J Biol Chem. In press
Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO (2003) Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 278: 1363–1371
Fuller N, Rand RP (2001) The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys J. 81: 243–254
Galazka A, Gasse F (1995) The present status of tetanus and tetanus vaccination. Curr. Top. Microbiol. Immunol. 195: 31–53
Gill DM (1982) Bacterial toxins: a table of lethal amounts. Microbiol. Rev. 46: 86–94
Hamilton JA (2003) Fast flip-flop of cholesterol and fatty acids in membranes: implications for membrane transport proteins. Curr. Opin. Lipidol. 14: 263–271
Harris JB (1997) Toxic phospholipases in snake venom: an introductory review. Symp. zool. Soc. Lond. 70: 235–250
Harris JB, Grubb BD, Maltin CA, Dixon R. (2000) The neurotoxicity of the venom phospholipases A(2), notexin and taipoxin. Exp. Neurol. 161: 517–526
Howard BD, Gundersen CB (1980) Effects and mechanisms of polypeptide neurotoxins that act presynaptically. Annu. Rev. Pharmacol. Toxicol. 20: 307–336
Howard BD, Wu WC (1976) Evidence that beta-bungarotoxin acts at the exterior of nerve terminals. Brain Res. 103: 190–192
Huang X, Wheeler MB, Kang YH, Sheu L, Luckacs GL, Trimble WS, Gaisano HY (1998) Truncated SNAP-25 (1–197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 121060–121070
Hughes R, Whaler BC(1962) Influence of nerve-endings activity and of drugs on the rate of paralysis of rat diaphragm preparations by Clostridium botulinum type A toxin. J. Physiol. (Lond) 160: 221–233
Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112: 519–533
Kamenskaya MA, Thesleff S (1974) The neuromuscular blocking action of an isolated toxin from the elapid (Oxyuranus scutellactus). Acta Physiol. Scand. 90: 716–724
Kelly RB, Brown BR (1974) Biochemical and physiological properties of a purified snake venom neurotoxin which acts presynaptically. J. Neurobiol. 5: 135–150
Kelly RB, Oberg SG, Strong PN, Wagner GM (1976) beta-Bungarotoxin, a phospholipase that stimulates transmitter release. Cold Spring Harb. Symp. Quant. Biol. 40: 117–125
Kielian M, Rey FA (2006) Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev.Microbiol. 4: 67–76
Kini RM (1997) Venom PhospholipaseA2 Enzymes. JohnWiley & Sons, Chichester
Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS (2000) Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associatedcalcium-binding protein 49 viaNP1 andNP2. J. Biol. Chem. 275: 17786–17792
Koriazova LK, Montal M (2003) Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 10: 13–18
Kularatne SA (2002) Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996–98. Postgrad. Med. J. 78: 276–280
Kwong PD, Mcdonald NQ, Sigler PB, Hendrickson WA (1995) Structure of beta 2-bungarotoxin: potassium channel binding by Kunitz modules and targeted phospholipase action. Structure 3: 1109–1119
Lacy DB, Tepp W, Cohen AC, Dasgupta BR, Stevens RC (1998) Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nature Struct. Biol. 5: 898–902
Lalli G, Bohnert S, Deinhardt K, Verastegui C, Schiavo G (2003) The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol. 11: 431–437
Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T (2006) The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 580:2011–2014
McArthur M, Atshaves B, Frolov A, Foxworth W, Kier A, Schroeder F (1999) Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res.: 1371–1383
Major RH (1945) Classic descriptions of disease. Springfield, IL: Charles C. Thomas
Montecucco C (1986) How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 11: 315–317
Montecucco C, Molgo J (2005) Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 5: 274–279
Montecucco C, Rossetto O (2000) How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem. Sci. 25: 266–270
Montecucco C, Rossetto O, Schiavo G (2004) Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 195: 221–241
Montecucco C, Schiavo G, Gao Z, Bauerlein E, Boquet P, Dasgupta BR (1988) Interaction of botulinum and tetanus toxins with the lipid bilayer surface. Biochem. J. 251: 379–383
Montecucco C, Schiavo G Dasgupta BR(1989) Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem. J. 259: 47–53
Montecucco C, Schiavo G, Pantano S (2005) SNARE complexes and neuroexocytosis: how many, how close? Trends Biochem. Sci. 30: 367–372
Neale EA, Bowers LM, Jia M, Bateman KE, Williamson LC, 1999. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J Cell Biol. 147: 1249–1260
Neco P, Rossetto O, Gil A, Montecucco C, Gutierrez LM (2003) Taipoxin induces Factin fragmentation and enhances release of catecholamines in bovine chromaffin cells. J. Neurochem. 85: 329–337
Ng RH, Howard BD (1978) Degenergization of nerve terminals by beta-bungarotoxin. Biochemistry 17: 4978–4986
Ng RH, Howard BD (1980)Mitochondria and sarcoplasmic reticulum as model targets for neurotoxic and myotoxic phospholipasesA2. Proc. Nat. Acad. Sci. USA 77: 1346–1350
Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sato K, Sekiguchi M, Takahashi M, Kozaki S (1996) The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 378: 253–257
Payling-Wright G (1955) The neurotoxins of Clostridium botulinum and Clostridium tetani. Pharmacol. Rev. 7: 413–465
Pearson JA, Tyler MI, Retson KV, Howden ME (1993) Studies on the subunit structure of textilotoxin, a potent presynaptic neurotoxin from the venom of the australian common brown snake (pseudonaja textilis). 3. the complete amino-acid sequences of all the subunits. Biochim Biophys Acta, 1161: 223–229
Petrovic U, Sribar J, Paris A, Rupnik M, Krzan M, Vardjan N, Gubensek F, Zorec R, Krizaj I (2004) Ammodytoxin, a neurotoxic secreted phospholipase A(2), can act in the cytosol of the nerve cell. Biochem. Biophys. Res. Commun. 324: 981–985
Prasarnpun S, Walsh J, Harris JB (2004) Beta-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology 47: 304–314
Prasarnpun S, Walsh J, Awad SS, Harris JB (2005) Envenoming bites by kraits: the biological basis of treatment-resistant neuromuscular paralysis. Brain., 128: 2987–2996
Puhar A, Johnson EA, Rossetto O, Montecucco C (2004) Comparison of the pH-induced conformational rearrangement of different clostridial neurotoxins. Biochem.Biophys.Res. Commun. 319: 66–71
Pumplin DW, Reese TS (1977)Action of brown widow spider venom and botulinum toxin on the frog neuromuscular junction examined with the freeze-fracture technique. J. Physiol. 273: 443–457
Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle Ad, Rossetto O, Schiavo G, Montecucco C (2005) Equivalent Effects of Snake PLA2 Neurotoxins and Lysophospholipid-Fatty Acid Mixtures. Science 310: 1678–1680
Rigoni M, Schiavo G, Weston AE, Caccin P, Allegrini F, Pennuto M, Valtorta F, Montecucco C, Rossetto O (2004) Snake presynaptic neurotoxins with phospholipase A2 activity induce puntate swellings of neurites and exocytosis of synaptic vesicles. J. Cell Sci. 117: 3561–1570
Rossetto O, Montecucco C (2004) Clostridial neurotoxins. In: Proft T (ed.), Microbial Toxins.Molecular and Cellular Biology. Horizon Scientific Press, Norfolk, UK: 149–178
Rummel A, Bade S, Alves J, Bigalke H, Binz T (2003) Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J. Mol. Biol. 326:835–847
Rummel A, Karnath T, Henke T, Bigalke H, Binz T (2004) Synaptotagmin I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 279: 30865-30870
Schiavo G, Matteoli M, Montecucco C (2000) Neurotoxins Affecting Neuroexocytosis. Physiol. Rev. 80: 717–766
Simpson LL (2000) Identification of the characteristics that underlie botulinum toxin potency implications for designing novel drugs. Biochimie 82: 943–953
Simpson LL, Coffield JA, Bakry N (1994). Inhibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipaseA2 neurotoxins. J. Pharmacol. Exp. Ther. 269: 256–262
Simpson LL, Lautenslager GT, Kaiser II, Middlebrook JL (1993) Identification of the site at which phospholipase A2 neurotoxins localise to produce their neuromuscular blocking effects. Toxicon, 31: 13–26
Singh G, Gourinath S, Sharma S, Paramasivam M, Srinivasan A, Singh TP (2001) Sequence and crystal structure determination of a basic phospholipase A2 from common krait (Bungarus caeruleus) at 2.4 A resolution: identification and characterization of its pharmacological sites. J. Mol. Biol. 307: 1049–1059
Sribar J, Copic A, Paris A, Sherman NE, Gubensek F, Fox JW, Krizaj I (2001) A high affinity acceptor for phospholipase A2 with neurotoxic activity is a calmodulin. J. Biol. Chem. 276: 12493-12496
Sribar J, Copic A, Poljsak-Prijatelj M, Kuret J, Logonder U, Gubensek F, Krizaj I (2003a) R25 is an intracellular membrane receptor for a snake venom secretori phospholipase A(2). FEBS Lett. 553: 309–314
Sribar J, Sherman NE, Prijatelj P, Faure G, Gubensek F, Fox JW, Aitken A, Pungercar J, Krizaj I (2003b) The neurotoxic phospholipase A2 associates, through a non-phosphorylated binding motif, with 14-3-3 protein gamma and epsilon isoforms. Biochem. Biophys. Res. Commun. 302: 691–696
Swaminathan S, Eswaramoorthy S (2000) Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 7: 693–699
Tacket CO, Rogawski MA (1989) Botulism. In: Simpson LL (ed) Botulinum Neurotoxins and Tetanus Toxin, Academic Press, San Diego: 351–378
Theakston RD, Phillips RE, Warrell DA, Galagedera Y, Abeysekera DT, Dissanayaka P, De Silva A, Aloysius DJ (1990) Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkine antivenom. Trans. R. Soc. Trop. Med. Hyg. 84: 301–308
Warrell DA, Looareesuwan S, White NJ, Theakston RD, Warrell MJ, Kosakarn W, Reid HA (1983) Severe neurotoxic envenoming by the Malayan krait Bungarus candidus (Linnaeus): response to antivenom and anticholinesterase. Br. Med. J. 286: 678–680
Weinstein L (1973) Tetanus. N. Engl. J. Med. 289: 1293–1296
Westerlund B, Nordlund P, Uhlin U, Eaker D, Eklund H (1992) The three-dimensional structure of notexin, a presynaptic neurotoxic phospholipase A2 at 2.0 A resolution. FEBS Lett. 301: 159–164
Yang CC (1997) Chemical modification and functional sites of phospholipases A2, In:Kini RM (ed), Venom Phospholipase A2 Enzymes. John Wiley & Sons, Chichester: 185–204
Zimmerberg J, Chernomordik LV (2005) Neuroscience. Synaptic membranes bend to the will of a neurotoxin. Science 310: 1626–1627
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossetto, O., Morbiato, L., Caccin, P. et al. Tetanus, botulinum and snake presynaptic neurotoxins. Rend. Fis. Acc. Lincei 19, 173–188 (2008). https://doi.org/10.1007/s12210-008-0010-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-008-0010-z