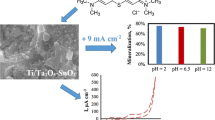
Abstract
A Pr-doped TiO2-NTs/SnO2-Sb electrode was prepared by a simple method, cyclic voltammetry(CV). The methyl orange(MO)aqueous solution was selected as a simulated wastewater. The ordered microstructural TiO2-NTs substrate was synthesized by an electrochemical method to obtain large specific surface area and high space utilization. The phase structure, electrode surface morphology and electrochemical properties of electrodes were characterized by XRD, SEM and electrochemical technology, respectively. The results showed that praseodymium oxide was successfully doped into the SnO2-Sb film by CV method. Due to the doped Pr, the oxygen evolution potential increased from 2.25 V to 2.40 V. The degradation of MO was investigated by UV-vis. The C t /C 0 (φ) was studied as a function to obtain the optimal parameters, such as the amount of doped Pr, current density and initial dye concentration. In addition, the degradation process followed pseudo-first-order reaction kinetics and the rate constant was 0.099 3 min-1. The result indicated that the introduction of Pr reduced the formation of oxygen vacancies or enhanced the formation of adsorbed hydroxyl radical groups on the surface, thus leading to better activity and stability.
Similar content being viewed by others
References
Xu L, Zhao H Z, Shi S Y et al. Electrolytic treatment of CI Acid Orange 7 in aqueous solution using a threedimensional electrode reactor [J]. Dyes and Pigments, 2008, 77(1): 158–164.
Rauf M A, Meetani M A, Hisaindee S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals [J]. Desalination, 2011, 276(1/2/3): 13–27.
Bisschops I, Spanjers H. Literature review on textile wastewater characterisation [J]. Environmental Technology, 2003, 24(11): 1399–1411.
Rodrigues C S D, Madeira L M. Treatment of textile effluent by chemical(Fenton’s Reagent)and biological (sequencing batch reactor)oxidation [J]. Journal of Hazardous Materials, 2009, 172(2/3): 1551–1559.
Glaze W H. Drinking-water treatment with ozone [J]. Environmental Science & Technology, 1987, 21(3): 224–230.
Rodgers J D, Bunce N J. Electrochemical treatment of 2, 4, 6-trinitrotoluene and related compounds [J]. Environmental Science & Technology, 2001, 35(2): 406–410.
Msindo Z S, Sibanda V, Potgieter J H. Electrochemical and physical characterization of lead-based anodes in comparison to Ti-(70%,)IrO2/(30%,)Ta2O5 dimensionally stable anodes for use in copper electrowinning [J]. Journal of Applied Electrochemistry, 2010, 40(3): 691–699.
Liu Y, Liu H L. Comparative studies on the electrocatalytic properties of modified PbO2 anodes [J]. Electrochimica Acta, 2008, 53(16): 5077–5083.
Costa C R, Montilla F, Morallon E et al. Electrochemical oxidation of acid black 210 dye on the boron-doped diamond electrode in the presence of phosphate ions: Effect of current density, pH, and chloride ions [J]. Electrochimica Acta, 2009, 54(27): 7048–7055.
Abaci S, Tamer U, Pekmez K et al. Electrosynthesis of benzoquinone from phenol on a and ß surfaces of PbO2 [J]. Electrochimica Acta, 2005, 50(18): 3655–3659.
Cui Y H, Feng Y J, Liu Z Q. Influence of rare earths doping on the structure and electro-catalytic performance of Ti/Sb-SnO2 electrodes [J]. Electrochimica Acta, 2009, 54(21): 4903–4909.
Kong J T, Shi S Y, Kong L C et al. Preparation and characterization of PbO2 electrodes doped with different rare earth oxides [J]. Electrochimica Acta, 2007, 53(4): 2048–2054.
Zhou X L, Ye Z G, Hua X Z et al. Electrocatalytic activity and stability of Ti/IrO2+MnO2 anode in 0. 5 M NaCl solution [J]. Journal of Solid State Electrochemistry, 2010, 14(7): 1213–1219.
Wang Y H, Li G, Chen Q Y et al. Effect of drying periods on antimony-doped tin dioxide-coated titanium electrode [J]. Journal of Solid State Electrochemistry, 2013, 17(7): 1985–1989.
Murakami Y, Kondo T, Shimoda Y et al. Effects of rare earth chlorides on the preparation of porous ruthenium oxide electrodes [J]. Journal of Alloys and Compounds, 1996, 239(2): 111–113.
Yang X P, Zou R Y, Huo F et al. Preparation and characterization of Ti/SnO2-Sb2O3-Nb2O5/PbO2 thin film as electrode material for the degradation of phenol [J]. Journal of Hazardous Materials, 2009, 164(1): 367–373.
Fan C M, Hua B, Wang Y et al. Preparation of Ti/SnO2-Sb2O4 photoanode by electrodeposition and dip coating for PEC oxidations [J]. Desalination, 2009, 249(2): 736–741.
Ding H Y, Feng Y J, Liu J F. Preparation and properties of Ti/SnO2-Sb2O5 electrodes by electrodeposition [J]. Materials Letters, 2007, 61(27): 4920–4923.
Wang F W, Li S D, Xu M et al. Effect of electrochemical modification method on structures and properties of praseodymium doped lead dioxide anodes [J]. Journal of the Electrochemical Society, 2013, 160(2): D53-D59.
Zhao G H, Lei Y Z, Zhang Y G et al. Growth and favorable bioelectrocatalysis of multishaped nanocrystal Au in vertically aligned TiO2 nanotubes for hemoprotein [J]. The Journal of Physical Chemistry C, 2008, 112(38): 14786–14795.
An H, Cui H, Zhang W Y et al. Fabrication and electrochemical treatment application of a microstructured TiO2-NTs/Sb-SnO2/PbO2 anode in the degradation of CI Reactive Blue 194(RB 194)[J]. Chemical Engineering Journal, 2012, 209: 86–93.
Wang Y H, Ma J, Ji F et al. Structural and photoluminescence characters of SnO2: Sb films deposited by RF magnetron sputtering [J]. Journal of Luminescence, 2005, 114(1): 71–76.
Rajaa I A, Manuel V, Abdlrani Y et al. Rapid decolourization and mineralization of the azo dye CI Acid Red 14 by heterogeneous Fenton reaction [J]. Journal of Hazardous Materials, 2011, 186(1): 745–750.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No. 20706041) and the Natural Science Foundation of Tianjin (No. 09JCYBJC06500)
Rights and permissions
About this article
Cite this article
Wang, Y., Chen, Y., Zhu, H. et al. Preparation and electrochemical application of praseodymium modified TiO2-NTs/SnO2-Sb anode by cyclic voltammetry method. Trans. Tianjin Univ. 22, 247–253 (2016). https://doi.org/10.1007/s12209-016-2727-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-016-2727-6