Abstract
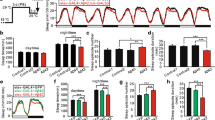
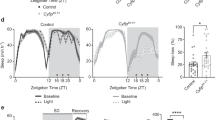
Amyloid precursor protein (APP) can generate neurotoxic β-amyloid 42 (Aβ42) by proteolytic process, which plays a crucial role in the pathogenesis of Alzheimer’s disease (AD). Individuals with mild to moderate AD exhibit sleep disturbance, even before the onset of AD. The purpose of this study is to verify the effect of APP on sleep behavior by using an APP overexpressing Drosophila AD model. APP-overexpressed flies were grouped by age, and their sleep amounts were monitored. Our results demonstrated that APP overexpression had no impacts on sleep amounts in young (4–7 days after eclosion, 4–7AE) flies. However, APP overexpression contributed to lower day and total sleep amounts in the middle-aged (11–14AE) flies. Moreover, old-aged (40AE) flies with overexpressing APP exhibited increased number of sleep bouts and decreased sleep time, indicating sleep fragmentation in these flies. Our results indicated that overexpression of APP in neurons has distinct effects on sleep behavior at different ages, but the specific mechanisms underlying the sleep regulation by APP are needed for further study. In addition, our data also suggest that sleep disturbance in AD animals can be caused by APP expression alterations, which provide a potential treating target for sleep intervention and therapy for AD patients.
Similar content being viewed by others
References
WYSS-CORAY T. Ageing, neurodegeneration and brain rejuvenation [J]. Nature, 2016, 539(7628): 180–186.
CANTER R G, PENNEY J, TSAI L H. The road to restoring neural circuits for the treatment of Alzheimer’s disease [J]. Nature, 2016, 539(7628): 187–196.
FARCA LUNA A J, PERIER M, SEUGNET L. Amyloid precursor protein in Drosophila glia regulates sleep and genes involved in glutamate recycling [J]. The Journal of Neuroscience, 2017, 37(16): 4289–4300.
ZHUANG L M, PENG F, HUANG Y Y, et al. CHIP modulates APP-induced autophagy-dependent pathological symptoms in Drosophila [J]. Aging Cell, 2020, 19(2): e13070.
CHEN K P, LU H X, GAO T L, et al. Synergic interaction between amyloid precursor protein and neural cell adhesion molecule promotes neurite outgrowth [J]. Oncotarget, 2016, 7(12): 14199–14206.
MOE K E, VITIELLO M V, LARSEN L H, et al. Sleep/wake patterns in Alzheimer’s disease: Relationships with cognition and function [J]. Journal of Sleep Research, 1995, 4(1): 15–20.
SONG Q, PING Y. Aβ42 differently regulates aggression and courtship behaviors in Drosophila [J]. Journal of Fuzhou University (Natural Science Edition), 2017, 45(2): 296–300 (in Chinese).
FENG G, PANG J, YI X, et al. Down-regulation of KV4 channel in Drosophila mushroom body neurons contributes to Aβ42-induced courtship memory deficits [J]. Neuroscience, 2018, 370: 236–245.
JU Y E S, LUCEY B P, HOLTZMAN D M. Sleep and Alzheimer disease pathology: A bidirectional relationship [J]. Nature Reviews Neurology, 2014, 10(2): 115–119.
POLLAK C P, PERLICK D. Sleep problems and institutionalization of the elderly [J]. Journal of Geriatric Psychiatry and Neurology, 1991, 4(4): 204–210.
VITIELLO M V, BORSON S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment [J]. CNS Drugs, 2001, 15(10): 777–796.
MCCURRY S M, ANCOLI-ISRAEL S. Sleep dysfunction in Alzheimer’s disease and other dementias [J]. Current Treatment Options in Neurology, 2003, 5(3): 261–272.
BLIWISE D L. Sleep disorders in Alzheimer’s disease and other dementias [J]. Clinical Cornerstone, 2004, 6(Sup 1A): S16–S28.
SONG Q, FENG G, HUANG Z H, et al. Aberrant axonal arborization of PDF neurons induced by Aβ42-mediated JNK activation underlies sleep disturbance in an Alzheimer’s model [J]. Molecular Neurobiology, 2017, 54(8): 6317–6328.
GUREVICIUS K, LIPPONEN A, TANILA H. Increased cortical and thalamic excitability in freely moving APPswe/PS1dE9 mice modeling epileptic activity associated with Alzheimer’s disease [J]. Cerebral Cortex, 2013, 23(5): 1148–1158.
JIN N X, LIPPONEN A, KOIVISTO H, et al. Increased cortical beta power and spike-wave discharges in middle-aged APP/PS1 mice [J]. Neurobiology of Aging, 2018, 71: 127–141.
DUBOWY C, SEHGAL A. Circadian rhythms and sleep in Drosophila melanogaster [J]. Genetics, 2017, 205(4): 1373–1397.
PITMAN J L, MCGILL J J, KEEGAN K P, et al. A dynamic role for the mushroom bodies in promoting sleep in Drosophila [J]. Nature, 2006, 441(7094): 753–756.
JOINER W J, CROCKER A, WHITE B H, et al. Sleep in Drosophila is regulated by adult mushroom bodies [J]. Nature, 2006, 441(7094): 757–760.
MORAN M, LYNCH C A, WALSH C, et al. Sleep disturbance in mild to moderate Alzheimer’s disease [J]. Sleep Medicine, 2005, 6(4): 347–352.
HAZRA A, CORBETT B F, YOU J C, et al. Corticothalamic network dysfunction and behavioral deficits in a mouse model of Alzheimer’s disease [J]. Neurobiology of Aging, 2016, 44: 96–107.
ZHANG J, PING Y. Regulation of lifespan by jet gene and jet lag in Drosophila [J]. Journal of Fudan University (Natural Science), 2019, 58(2): 199–209 (in Chinese).
BUHL E, HIGHAM J P, HODGE J J L. Alzheimer’s disease-associated tau alters Drosophila circadian activity, sleep and clock neuron electrophysiology [J]. Neurobiology of Disease, 2019, 130: 104507.
FERNANDEZ-FUNEZ P, DE MENA L, RINCONLIMAS D E. Modeling the complex pathology of Alzheimer’s disease in Drosophila [J]. Experimental Neurology, 2015, 274: 58–71.
FENG G, ZHANG J, LI M Z, et al. Control of sleep onset by Shal/Kv4 channels in Drosophila circadian neurons [J]. The Journal of Neuroscience, 2018, 38(42): 9059–9071.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item
the National Natural Science Foundation of China (No. 81970999), and the Shanghai Rising Star Project (No. 19QA1404900)
Rights and permissions
About this article
Cite this article
Li, M., Ping, Y. Regulation of Sleep Behavior by Overexpression of Amyloid Precursor Protein in Drosophila Neurons. J. Shanghai Jiaotong Univ. (Sci.) 26, 63–68 (2021). https://doi.org/10.1007/s12204-021-2261-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-021-2261-0