Abstract
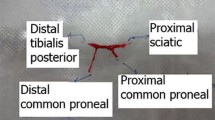
The present study presents a peripheral nerve injury animal model for evaluation of an implantable electrical stimulator we designed. The evaluation was confirmed by ethological, electrophysiological and histologic study. Twenty New Zealand rabbits were used. The left sciatic nerve was crushed with a micro-vessel clamp in all rabbits, and the stimulators were implanted in ten rabbits. The other ten rabbits were in a control group. As compared with the implantation group, 3 and 6 weeks after operation, the histology showed the typical pathologic atrophy by hematoxylin-eosin (HE), the motor nerve conduction velocity (MCV) was found significantly slowed and the axon of crushed nerve at distal portion exhibited breakdown in the control group. These results reveal that the implantable electrical stimulator was effective and was suitable for implantation in an animal model.
Similar content being viewed by others
References
English A W, Schwartz G, Meador W, et al. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling [J]. Dev Neurobiol, 2007, 67(2): 158–172.
Geremia N M, Gordon T, Brushart T M, et al. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression [J]. Exp Neurol, 2007, 205(2): 347–359.
Zhang Z, Rouabhia M, Wang Z, et al. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration [J]. Artif Organs, 2007, 31(1): 13–22.
Yamada M, Tanemura K, Okada S, et al. Electrical stimulation modulates fate determination of differentiating embryonic stem cells [J]. Stem Cells, 2007, 25(3): 562–570.
Zealear D L, Rodriguez R J, Kenny T, et al. Electrical stimulation of a denervated muscle promotes selective reinnervation by native over foreign motoneurons [J]. J Neurophysiol, 2002, 87(4): 2195–2199.
Al-Majed A A, Tam S L, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons [J]. Cell Mol Neurobiol, 2004, 24(3): 379–402.
Meissner W, Gross C E, Harnack D, et al. Deep brain stimulation for Parkinson’s disease: Potential risk of tissue damage associated with external stimulation [J]. Ann Neurol, 2004, 55(3): 449–450.
Chiou Y H, Luh J J, Chen S C, et al. Patient-driven loop control for hand function restoration in a non-invasive functional electrical stimulation system [J]. Disabil Rehabil, 2008, 30(19): 1499–1505.
Lundborg G. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance [J]. J Hand Surg [Am], 2000, 25(3): 391–414.
Naff N J, Ecklund J M. History of peripheral nerve surgery techniques [J]. Neurosurg Clin North Am, 2001, 12(1): 197–209.
Dvali L, Mackinnon S E. Nerve repair, grafting, and nerve transfers [J]. Clin Plast Surg, 2003, 30(2): 203–221.
Tong K C, Lo S K, Cheing G L. Alternating frequencies of transcutaneous electric nerve stimulation: Does it produce greater analgesic effects on mechanical and thermal pain thresholds [J]. Arch Phys Med Rehabil, 2007, 88(10): 1344–1349.
Malm-Buatsi E, Nepple K G, Boyt M A, et al. Efficacy of transcutaneous electrical nerve stimulation in children with overactive bladder refractory to pharmacotherapy [J]. Urology, 2007, 70(5): 980–983.
Robaina F, Clavo B. Spinal cord stimulation in the treatment of post-stroke patients: Current state and future directions [J]. Acta Neurochir Suppl, 2007, 97(Sup 1): 277–282.
Hamid S, Hayek R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview [J]. Eur Spine J, 2008, 17(9): 1256–1269.
Hamani C, Schwalb J M, Rezai A R, et al. Deep brain stimulation for chronic neuropathic pain: Long-term outcome and the incidence of insertional effect [J]. Pain, 2006, 25(1–2): 188–196.
Beer G M, Steurer J, Meyer V E. Standardizing nerve crushes with a non-serrated clamp [J]. J Reconstr Microsurg, 2001, 17(7): 531–534.
Varejao A S, Cabrita A M, Meek M F, et al. Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp [J]. J Neurotrauma, 2004, 21(11): 1652–1670.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: the Shanghai-Philips Research & Development Fund of China (No. 06SP07001) and Shanghai Rising-Star Program (No. 08QH14015)
Rights and permissions
About this article
Cite this article
Rui, By., Zeng, Bf., Wang, Jw. et al. Using the implantable electrical stimulator for peripheral nerve rehabilitation: A study in an animal model. J. Shanghai Jiaotong Univ. (Sci.) 14, 635–640 (2009). https://doi.org/10.1007/s12204-009-0635-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-009-0635-9