Abstract
Objectives
High-fat and -cholesterol diet (HFC) induced fibrotic steatohepatitis in stroke-prone spontaneously hypertensive rat (SHRSP) 5/Dmcr, the fifth substrain from SHRSP, by dysregulating bile acid (BA) kinetics. This study aimed to clarify the histopathological and BA kinetic differences in HFC-induced fibrosis between SHRSP5/Dmcr and SHRSP.
Methods
Ten-week-old male SHRSP5/Dmcr and SHRSP were randomly allocated to groups and fed with either control diet or HFC for 2 and 8 weeks. The liver histopathology, biochemical features, and molecular signaling involved in BA kinetics were measured.
Results
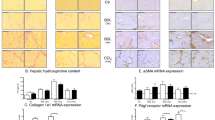
HFC caused more severe hepatocyte ballooning, macrovesicular steatosis and fibrosis in SHRSP5/Dmcr than in SHRSP. It was noted that fibrosis was disproportionately formed in retroperitoneal side of both strains. As for BA kinetics, HFC greatly increased the level of Cyp7a1 and Cyp7b1 to the same degree in both strains at 8 weeks, while multidrug resistance-associated protein 3 was greater in SHRSP5/Dmcr than SHRSP. The diet decreased the level of bile salt export pump by the same degree in both strains, while constitutive androstane receptor, pregnane X receptor, and UDP-glucuronosyltransferase activity more prominent in SHRSP5/Dmcr than SHRSP at 8 weeks. In the fibrosis-related genes, only expression of collagen, type I, alpha 1 mRNA was greater in SHRSP5/Dmcr than SHRSP.
Conclusions
The greater progression of fibrosis in SHRSP5/Dmcr induced by HFC may be due to greater suppression of UDP-glucuronosyltransferase activity detoxifying toxicants, such as hydrophobic BAs.






Similar content being viewed by others
Abbreviations
- αSMA:
-
Alpha smooth muscle actin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BA:
-
Bile acid
- BSEP:
-
Bile salt export pump
- CA:
-
Cholic acid
- CAR:
-
Constitutive androstane receptor
- CDCA:
-
Chenodeoxycholic acid
- COL1a1:
-
Collagen, type I, alpha 1
- CV:
-
Central vein
- CYP27A1:
-
Cholesterol 27-hydroxylase
- CYP7A1:
-
Cholesterol 7α-hydroxylase
- CYP7B1:
-
Oxysterol 7α-hydroxylase
- CYP8B1:
-
Sterol 12α-hydroxylase
- EVG:
-
Elastic Van Gieson
- FXR:
-
Farnesoid X receptor
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GGT:
-
Γ-Glutamyl transpeptidase
- H&E:
-
Hematoxylin and eosin
- HFC:
-
High-fat and -cholesterol diet
- MRP:
-
Resistance-associated protein
- MMP-2:
-
Matrix metalloproteinase-2
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- PDGFβR:
-
Platelet-derived growth factor receptor, β polypeptide
- PXR:
-
Pregnane X receptor
- SHRSP:
-
Stroke-prone spontaneously hypertensive rat
- SULT:
-
Sulfotransferase
- TC:
-
Total cholesterol
- UGT:
-
UDP-glucuronosyltransferase
References
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–112.
Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–16.
Yasutake K, Nakamuta M, Shima Y, Ohyama A, Masuda K, Haruta N, et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol. 2009;44:471–7.
Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–42.
Haslewood GA. The biological significance of chemical differences in bile salts. Biol Rev Camb Philos Soc. 1964;39:537–74.
Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–71.
Jia X, Naito H, Yetti H, Tamada H, Kitamori K, Hayashi Y, et al. Dysregulated bile acid synthesis, metabolism and excretion in a high fat-cholesterol diet-induced fibrotic steatohepatitis in rats. Dig Dis Sci. 2013;58:2212–22.
Jia X, Suzuki Y, Naito H, Yetti H, Kitamori K, Hayashi Y, et al. A possible role of chenodeoxycholic acid and glycine-conjugated bile acids in fibrotic steatohepatitis in a dietary rat model. Dig Dis Sci. 2014;59:1490–501.
Zollner G, Trauner M. Nuclear receptors as therapeutic targets in cholestatic liver diseases. Br J Pharmacol. 2009;156:7–27.
Kitamori K, Naito H, Tamada H, Kobayashi M, Miyazawa D, Yasui Y, et al. Development of novel rat model for high-fat and high-cholesterol diet-induced steatohepatitis and severe fibrosis progression in SHRSP5/Dmcr. Environ Health Prev Med. 2012;17:173–82.
Jia X, Naito H, Yetti H, Tamada H, Kitamori K, Hayashi Y, et al. The modulation of hepatic adenosine triphosphate and inflammation by eicosapentaenoic acid during severe fibrotic progression in the SHRSP5/Dmcr rat model. Life Sci. 2012;90:934–43.
Nakajima T, Naito H. Mechanism analysis and prevention of pathogenesis of nonalcoholic steatohepatitis. Nihon Eiseigaku Zasshi. 2015;70:197–204 (in Japanese).
Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, et al. Human udp-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos. 2004;32:413–23.
Lee CH, Ito Y, Yanagiba Y, Yamanoshita O, Kim H, Zhang SY, et al. Pyrene-induced CYP1A2 and SULT1A1 may be regulated by CAR and not by AhR. Toxicology. 2007;238:147–56.
Suzuki Y, Kaneko R, Nomura M, Naito H, Kitamori K, Nakajima T, et al. Simple and rapid quantitation of 21 bile acids in rat serum and liver by UPLC-MS-MS: effect of high fat diet on glycine conjugates of rat bile acids. Nagoya J Med Sci. 2013;75:57–71.
Prosser CC, Yen RD, Wu J. Molecular therapy for hepatic injury and fibrosis: where are we? World J Gastroenterol. 2006;12:509–15.
Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–9.
Trottier J, Milkiewicz P, Kaeding J, Verreault M, Barbier O. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3:212–22.
Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–30.
Greco AV, Mingrone G. Serum bile acid concentrations in mild liver cirrhosis. Clin Chim Acta. 1993;221:183–9.
Vlahcevic ZR, Goldman M, Schwartz CC, Gustafsson J, Swell L. Bile acid metabolism in cirrhosis. VII. Evidence for defective feedback control of bile acid synthesis. Hepatology. 1981;1:146–50.
Trottier J, Verreault M, Grepper S, Monte D, Belanger J, Kaeding J, et al. Human UDP-glucuronosyltransferase (UGT)1A3 enzyme conjugates chenodeoxycholic acid in the liver. Hepatology. 2006;44:1158–70.
Trottier J, Perreault M, Rudkowska I, Levy C, Dallaire-Theroux A, Verreault M, et al. Profiling serum bile acid glucuronides in humans: gender divergences, genetic determinants, and response to fenofibrate. Clin Pharmacol Ther. 2013;94:533–43.
Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5.
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8.
Bock KW. Functions and transcriptional regulation of adult human hepatic UDP-glucuronosyl-transferases (UGTs): mechanisms responsible for interindividual variation of UGT levels. Biochem Pharmacol. 2010;80:771–7.
Stedman CA, Liddle C, Coulter SA, Sonoda J, Alvarez JG, Moore DD, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–8.
Hanada K, Nakai K, Tanaka H, Suzuki F, Kumada H, Ohno Y, et al. Effect of nuclear receptor downregulation on hepatic expression of cytochrome P450 and transporters in chronic hepatitis C in association with fibrosis development. Drug Metab Pharmacokinet. 2012;27:301–6.
Akita H, Suzuki H, Sugiyama Y. Sinusoidal efflux of taurocholate is enhanced in Mrp2-deficient rat liver. Pharm Res. 2001;18:1119–25.
Hirohashi T, Suzuki H, Ito K, Ogawa K, Kume K, Shimizu T, et al. Hepatic expression of multidrug resistance-associated protein-like proteins maintained in eisai hyperbilirubinemic rats. Mol Pharmacol. 1998;53:1068–75.
Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem. 1999;274:15181–5.
Brunt EM. Nonalcoholic steatohepatitis. Semin Liver Dis. 2004;24:3–20.
Yetti H, Naito H, Jia X, Shindo M, Taki H, Tamada H, et al. High-fat-cholesterol diet-induced mainly necrosis in fibrotic steatohepatitis rats by suppressing caspase activity. Life Sci. 2013;93:673–80.
Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284:9947–54.
Hui AY, Chan HL, Wong VW, Liew CT, Chim AM, Chan FK, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol. 2005;100:616–23.
Shi Y, Guo Q, Xia F, Dzyubak B, Glaser KJ, Li Q, et al. MR elastography for the assessment of hepatic fibrosis in patients with chronic hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology. 2014;273:88–98.
Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62.
Fu ZD, Csanaky IL, Klaassen CD. Gender-divergent profile of bile acid homeostasis during aging of mice. PLoS One. 2012;7:e32551.
Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–95.
Acknowledgments
The work has been supported by Grants-in-Aid for Scientific Research (B. 23390161, C. 25460797), Grants-in-Aid for Young Scientists (B. 22790543, Start-up. 21890098) and Uehara Memorial Foundation in 2009 (Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naito, H., Jia, X., Yetti, H. et al. Importance of detoxifying enzymes in differentiating fibrotic development between SHRSP5/Dmcr and SHRSP rats. Environ Health Prev Med 21, 368–381 (2016). https://doi.org/10.1007/s12199-016-0539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12199-016-0539-x