Abstract
Introduction
Metabolic strategies in different microenvironments can affect cancer metabolic adaptation, ultimately influencing the therapeutic response. Understanding the metabolic alterations of cancer cells in different microenvironments is critical for therapeutic success.
Methods
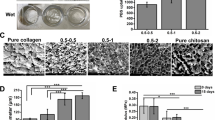
In this study, we cultured non-small cell lung cancer cells in three different microenvironments (two-dimensional (2D) plates, soft elastic three-dimensional (3D) porous 2 wt% scaffolds, and stiff elastic 3D porous 4 wt% scaffolds) to investigate the effects of different matrix elasticity as well as 2D and 3D culture settings on the metabolic adaptation of cancer cells.
Results
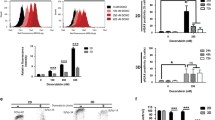
The results revealed that PGC-1α expression is sensitive to the elasticity of the 3D scaffold. PGC-1α expression was markedly increased in cancer cells cultured in stiff elastic 3D porous 4 wt% scaffolds compared with cells cultured in soft elastic 3D porous 2 wt% scaffolds or 2D plates, enhancing mitochondrial biogenesis and oxidative stress resistance of non-small cell lung cancer through increased reactive oxygen species (ROS) detoxification capacity. However, phosphofructokinase-1 (PFK-1) expression, a key rate-limiting enzyme in glycolysis, did not change significantly in the three microenvironments, indicating that microenvironments may not affect the early stage of glycolysis. Conversely, monocarboxylate transporter 1 (MCT1) expression in 3D culture was significantly reduced compared to 2D culture but without significant difference between soft and stiff scaffolds, indicating that MCT1 expression is more sensitive to the shape of the different cultures of 2D and 3D microenvironment surrounding cells but is unaffected by the scaffold elasticity.
Conclusions
Together, these results demonstrate that differences in the microenvironment of cancer cells profoundly impact their metabolic response.






Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- GAGs:
-
Glycosaminoglycans
- MCT1:
-
Monocarboxylate transporter
- PEC:
-
Polyacrylic complex
- PFK-1:
-
Phosphofructokinase-1
- ROS:
-
Reactive oxygen species
- PGC-1α:
-
Transcription coactivator peroxisome proliferator-activated receptor gamma
- SOD2:
-
Manganese superoxide dismutase
- SOD1:
-
Recombinant superoxide dismutase 1
- TBST:
-
Tris-buffered saline with TweenR 20
- TCA:
-
Tricarboxylic acid
- 3D:
-
Three-dimensional
- 2D:
-
Two-dimensional
References
Barbosa, I. A., N. G. Machado, A. J. Skildum, P. M. Scott, and P. J. Oliveira. Mitochondrial remodeling in cancer metabolism and survival: potential for new therapies. Biochim. Biophys. Acta (BBA). 238–254:2012, 1826.
Beloueche-Babari, M., et al. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 77:5913–5924, 2017.
Bissell, M. J., and W. C. Hines. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17:320–329, 2011.
Cazzaniga, M., and B. Bonanni. Relationship between metabolic reprogramming and mitochondrial activity in cancer cells. Understanding the anticancer effect of metformin and its clinical implications. Anticancer Res. 35:5789–5796, 2015.
Cheng, G., et al. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 72:2634–2644, 2012.
Cheng, G., J. Zielonka, D. McAllister, S. Tsai, M. B. Dwinell, and B. Kalyanaraman. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Br. J. Cancer. 111:85–93, 2014.
Cox, T. R., and J. T. Erler. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4:165–178, 2011.
Doench, J. G. Am I ready for CRISPR? A user’s guide to genetic screens. Nat. Rev. Genet. 19:67–80, 2018.
Fantin, V. R., J. St-Pierre, and P. Leder. Attenuation of LDH-a expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 9:425–434, 2006.
Faubert, B., et al. Lactate metabolism in human lung tumors. Cell. 171:358–371, 2017.
Florczyk, S. J., F. M. Kievit, K. Wang, A. E. Erickson, R. G. Ellenbogen, and M. Zhang. 3D porous chitosan–alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J. Mater. Chem. B. 4:6326–6334, 2016.
Gao, Y., K. Peng, and S. Mitragotri. Covalently crosslinked hydrogels via step-growth reactions: crosslinking chemistries, polymers, and clinical impact. Adv. Mater. 33:2006362, 2021.
Gargiulo, G., M. Serresi, M. Cesaroni, D. Hulsman, and M. Van Lohuizen. In vivo shRNA screens in solid tumors. Nat. Protoc. 9:2880–2902, 2014.
Gottlieb, E., and I. P. M. Tomlinson. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer. 5:857–866, 2005.
Halestrap, A. P. Monocarboxylic acid transport. Compr. Physiol. 3:1611–1643, 2013.
Handschin, C., et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J. Biol. Chem. 282:30014–30021, 2007.
Hutmacher, D. W. Biomaterials offer cancer research the third dimension. Nat. Mater. 9:90–93, 2010.
Jose, C., N. Bellance, and R. Rossignol. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim. Biophys. Acta (BBA). 552–561:2011, 1807.
Kirdponpattara, S., A. Khamkeaw, N. Sanchavanakit, P. Pavasant, and M. Phisalaphong. Structural modification and characterization of bacterial cellulose–alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 132:146–155, 2015.
Koppenol, W. H., P. L. Bounds, and C. V. Dang. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 11:325–337, 2011.
LeBleu, V. S., et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16:992–1003, 2014.
Li, Z., H. R. Ramay, K. D. Hauch, D. Xiao, and M. Zhang. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 26:3919–3928, 2005.
Luengo, A., D. Y. Gui, and M. G. Vander Heiden. Targeting metabolism for cancer therapy. Cell Chem. Biol. 24:1161–1180, 2017.
Marchiq, I., R. Le Floch, D. Roux, M.-P. Simon, and J. Pouyssegur. Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Res. 75:171–180, 2015.
Matoba, S., et al. p53 regulates mitochondrial respiration. Science (80-). 312:1650–1653, 2006.
McMillan, E. A., et al. Chemistry-first approach for nomination of personalized treatment in lung cancer. Cell. 173:864–878, 2018.
Michelakis, E. D., et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci. Transl. Med. 2:31ra34-31ra34, 2010.
Moreno-Sánchez, R., S. Rodriguez-Enriquez, A. Marin-Hernández, and E. Saavedra. Energy metabolism in tumor cells. FEBS J. 274:1393–1418, 2007.
Ozel, D., R. Y. Bayraktarli, and Z. U. Coskun. Re: Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J. Urol. 193:794–800, 2015.
Park, J. S., et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 578:621–626, 2020.
Samani, A., J. Zubovits, and D. Plewes. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 52:1565, 2007.
Sancho, P., D. Barneda, and C. Heeschen. Hallmarks of cancer stem cell metabolism. Br. J. Cancer. 114:1305–1312, 2016.
St-Pierre, J., et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 127:397–408, 2006.
Sullivan, L. B., and N. S. Chandel. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2:1–12, 2014.
Tasdogan, A., et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature. 577:115–120, 2020.
Vazquez, F., et al. PGC1$α$ expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 23:287–301, 2013.
Viswanathan, V. S., et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 547:453–457, 2017.
Wang, K., F. M. Kievit, S. J. Florczyk, Z. R. Stephen, and M. Zhang. 3D porous chitosan–alginate scaffolds as an in vitro model for evaluating nanoparticle-mediated tumor targeting and gene delivery to prostate cancer. Biomacromolecules. 16:3362–3372, 2015.
Warburg, O. On the origin of cancer cells. Science. 123:309–314, 1956.
Wellman, P., R.D. Howe, E. Dalton, and K.A. Kern. Breast tissue stiffness in compression is correlated to histological diagnosis. Harvard BioRobotics Lab. Tech. Rep. 1, 1999.
Xu, K., et al. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials.217:119311, 2019.
Yu, H., J. K. Mouw, and V. M. Weaver. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 21:47–56, 2011.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science under Grants-in-Aid for Scientific Research (S; No. 17H06146). Xiaorong Fu acknowledges the support of the China Scholarship Council (Grant Number 201906050131).
Author Contributions
XF: Investigation, Methodology, Visualization, Writing-original draft, Writing- review & editing. YK: Resources, Investigation, Methodology, Writing-review & editing. YT: Resources, Writing-review & editing. GS: Resources, Writing- review & editing. YJ: Conceptualization, Supervision, Writing- original draft, Writing- review & editing, Funding acquisition.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of interest
X.F., Y.K., Y.T., G.S., and Y.J. declare that they have no financial, professional, or personal conflict of interest.
Ethical Approval
No animal or human studies were carried out by the authors of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, X., Kimura, Y., Toku, Y. et al. Stiffer-Matrix-Induced PGC-1α Upregulation Enhanced Mitochondrial Biogenesis and Oxidative Stress Resistance in Non-small Cell Lung Cancer. Cel. Mol. Bioeng. 16, 69–80 (2023). https://doi.org/10.1007/s12195-022-00751-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00751-x