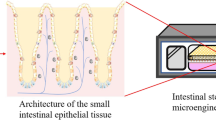
Abstract
Introduction
Life on Earth depends on oxygen; human tissues require oxygen signaling, whereas many microorganisms, including bacteria, thrive in anoxic environments. Despite these differences, human tissues and bacteria coexist in close proximity to each other such as in the intestine. How oxygen governs intestinal-bacterial interactions remains poorly understood.
Methods
To address to this gap, we created a dual-oxygen environment in a microfluidic device to study the role of oxygen in regulating the regulation of intestinal enzymes and proteins by gut bacteria. Two-layer microfluidic devices were designed using a fluid transport model and fabricated using soft lithography. An oxygen-sensitive material was integrated to determine the oxygen levels. The intestinal cells were cultured in the upper chamber of the device. The cells were differentiated, upon which bacterial strains, a facultative anaerobe, Escherichia coli Nissle 1917, and an obligate anaerobe, Bifidobacterium Adolescentis, were cultured with the intestinal cells.
Results
The microfluidic device successfully established a dual-oxygen environment. Of particular importance in our findings was that both strains significantly upregulated mucin proteins and modulated several intestinal transporters and transcription factors but only under the anoxic–oxic oxygen gradient, thus providing evidence of the role of oxygen on bacterial-epithelial signaling.
Conclusions
Our work that integrates cell and molecular biology with bioengineering presents a novel strategy to engineer an accessible experimental system to provide tailored oxygenated environments. The work could provide new avenues to study intestine-microbiome signaling and intestinal tissue engineering, as well as a novel perspective on the indirect effects of gut bacteria on tissues including tumors.






Similar content being viewed by others
Data Availability
The data and material are listed in supplementary documents.
Code Availability
Not applicable.
Abbreviations
- O2C−:
-
Culture condition with no control of oxygen
- O2C+:
-
Culture condition with oxygen control
- ECN:
-
E. coli Nissle 1917
- Bifido :
-
Bifidobacterium adolescentis (B. adolescentis)
- MOI:
-
Multiplicity of Infection of bacteria to cells
- CYP:
-
Cytochrome P450
- MUC2:
-
Mucin 2
References
Ambrose, N. S., M. Johnson, D. W. Burdon, and M. R. B. Keighley. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn’s disease surgery. BJS. 71:623–625, 1984.
Belenguer, A., S. H. Duncan, A. G. Calder, G. Holtrop, P. Louis, G. E. Lobley, and H. J. Flint. Two routes of metabolic cross-feeding between bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 72:3593–3599, 2006.
Belzer, C., and W. M. de Vos. Microbes inside—from diversity to function: the case of Akkermansia. ISME J. 6:1449–1458, 2012.
Brennan, M. D., M. L. Rexius-Hall, L. J. Elgass, and D. T. Eddington. Oxygen control with microfluidics. Lab Chip. 14:4305–4318, 2014.
Corrêa-Oliveira, R., J. L. Fachi, A. Vieira, F. T. Sato, and M. A. R. Vinolo. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5:e73, 2016.
Frank, D. N., A. L. St Amand, R. A. Feldman, E. C. Boedeker, N. Harpaz, and N. R. Pace. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci.. 104:13780–13785, 2007.
Glen, I. Pharmacokinetic variation. Anaesth. Intensive Care Med. 6:282–285, 2005.
Gorczyca, L., and L. M. Aleksunes. Transcription factor-mediated regulation of the BCRP/ABCG2 efflux transporter: a review across tissues and species. Expert Opin. Drug Metab. Toxicol. 16:239–253, 2020.
Haddish-Berhane, N., A. Farhadi, C. Nyquist, K. Haghighi, and A. Keshavarzian. SIMDOT-AbMe: microphysiologically based simulation tool for quantitative prediction of systemic and local bioavailability of targeted oral delivery formulations. Drug Metab. Dispos. 37:608–618, 2009.
Haiser, H. J., K. L. Seim, E. P. Balskus, and P. J. Turnbaugh. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes. 5:233–238, 2014.
Hentges DJ. Anaerobes: General Characteristics. In: Medical Microbiology, edited by S. Baron Galveston: University of Texas Medical Branch at Galveston. http://www.ncbi.nlm.nih.gov/books/NBK7638/. 1996. Accessed May 27, 2021.
Imlay, J. A. How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv. Microb. Physiol. 46:111–153, 2002.
Jalili-Firoozinezhad, S., F. S. Gazzaniga, E. L. Calamari, D. M. Camacho, C. W. Fadel, A. Bein, B. Swenor, B. Nestor, M. J. Cronce, A. Tovaglieri, O. Levy, K. E. Gregory, D. T. Breault, J. M. S. Cabral, D. L. Kasper, R. Novak, and D. E. Ingber. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3:520–531, 2019.
Johansson, M. E. V., and G. C. Hansson. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16:639–649, 2016.
Kelly, C. J., L. Zheng, E. L. Campbell, B. Saeedi, C. C. Scholz, A. J. Bayless, K. E. Wilson, L. E. Glove, D. J. Kominsky, A. Magnuson, T. L. Weir, S. F. Ehrentraut, C. Pickel, K. A. Kuhn, J. M. Lanis, V. Nguyen, C. T. Taylor, and S. P. Colgan. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 17:662–671, 2015.
Kim, R., P. J. Attayek, Y. Wang, K. L. Furtado, R. Tamayo, C. E. Sims, and N. L. Allbritton. An in vitro intestinal platform with a self-sustaining oxygen gradient to study the human gut/microbiome interface. Biofabrication. 12:015006, 2019.
Krayenbühl, J. C., S. Vozeh, M. Kondo-Oestreicher, and P. Dayer. Drug-drug interactions of new active substances: mibefradil example. Eur. J. Clin. Pharmacol. 55:559–565, 1999.
Li, Y., Q. Wang, X. Yao, and Y. Li. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor- and pregnane X receptor-mediated pathways. Eur. J. Pharmacol. 640:46–54, 2010.
Lin, J. H. Pharmacokinetic and pharmacodynamic variability: a daunting challenge in drug therapy. Curr. Drug Metab. 8:109–136, 2007.
Livak, K. J., and T. D. Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408, 2001.
Lukovac, S., C. Belzer, L. Pellis, B. J. Keijser, W. M. de Vos, R. C. Montijn, and G. Roeselers. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 5:e01438-14, 2014. https://doi.org/10.1128/mBio.01438-14.
Marrero, D., F. Pujol-Vila, D. Vera, G. Gabriel, X. Illa, A. Elizalde-Torrent, M. Alvarez, and R. Villa. Gut-on-a-chip: mimicking and monitoring the human intestine. Biosens. Bioelectron. 181:113156, 2021.
Mathan, V. I., J. Wiederman, J. F. Dobkin, and J. Lindenbaum. Geographic differences in digoxin inactivation, a metabolic activity of the human anaerobic gut flora. Gut. 30:971–977, 1989.
Matuskova, Z., E. Anzenbacherova, R. Vecera, H. Tlaskalova-Hogenova, M. Kolar, and P. Anzenbacher. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on Amidarone absorption in rats. PLoS ONE. 9:e8715, 2014.
Ortiz-Prado, E., J. F. Dunn, J. Vasconez, D. Castillo, and G. Viscor. Partial pressure of oxygen in the human body: a general review. Am. J. Blood Res. 9:1–14, 2019.
Ott, S. J., M. Musfeldt, D. F. Wenderoth, J. Hampe, O. Brant, U. R. Fölsch, K. N. Timmis, and S. Schreiber. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 53:685–693, 2004.
Paine, M. F., H. L. Hart, S. S. Ludington, R. L. Haining, A. E. Rettie, and D. C. Zeldin. The human intestinal cytochrome P450 “PIE.” Drug Metab. Dispos. Biol. Fate Chem. 34:880–886, 2006.
Paone, P., and P. D. Cani. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020. https://doi.org/10.1136/gutjnl-2020-322260.
Patel, J., K. Landers, H. Li, R. H. Mortimer, and K. Richard. Oxygen concentration regulates expression and uptake of transthyretin, a thyroxine binding protein, in JEG-3 choriocarcinoma cells. Placenta. 32:128–133, 2011.
Pikuleva, I. A., and M. R. Waterman. Cytochromes P450: roles in diseases. J. Biol. Chem. 288:17091–17098, 2013.
Saha, J. R., V. P. Butler Jr., H. C. Neu, and J. Lindenbaum. Digoxin-inactivating bacteria: identification in human gut flora. Science. 220:325–327, 1983.
Shah, Y. M. The role of hypoxia in intestinal inflammation. Mol. Cell Pediatr. 3:1, 2016.
Shah, P., T. Guo, D. D. Moore, and R. Ghose. Role of constitutive androstane receptor in Toll-like receptor-mediated regulation of gene expression of hepatic drug-metabolizing enzymes and transporters. Drug Metab. Dispos. Biol. Fate Chem. 42:172–181, 2014.
Shin, W., and H. J. Kim. Intestinal barrier dysfunction orchestrates the onset of inflammatory host–microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. 115:E10539–E10547, 2018.
Shugarts, S., and L. Z. Benet. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 26:2039–2054, 2009.
Sonnenborn, U. Escherichia coli strain Nissle 1917-from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 363:212, 2016.
Stillhart, C., K. Vučićević, P. Augustijns, A. W. Basit, H. Batchelor, T. R. Flanagan, I. Gesquiere, R. Greupink, D. Keszthelyi, M. Koskinen, C. M. Madla, C. Matthys, G. Miljuš, M. G. Mooij, N. Parrott, A.-L. Ungell, S. N. de Wildt, M. Orlu, S. Klein, and A. Müllertz. Impact of gastrointestinal physiology on drug absorption in special populations––an UNGAP review. Eur. J. Pharm. Sci. 147:105280, 2020.
Unden, G., S. Becker, J. Bongaerts, J. Schirawski, and S. Six. Oxygen regulated gene expression in facultatively anaerobic bacteria. Ant. Van Leeuwen. 66:3–22, 1994.
van Kessel, S. P., A. K. Frye, A. O. El-Gendy, M. Castejon, A. Keshavarzian, G. van Dijk, and S. E. Aidy. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 10:310, 2019.
Wang, C., T. Dang, J. Baste, A. A. Joshi, and A. Bhushan. A novel standalone microfluidic device for local control of oxygen tension for intestinal-bacteria interactions. FASEB J. 35:e21291, 2021.
Waring, M. J., J. Arrowsmith, A. R. Leach, P. D. Leeson, S. Mandrell, R. M. Owen, G. Pairaudeau, W. D. Pennie, S. D. Pickett, J. Wang, O. Wallace, and A. Weir. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 14:475–486, 2015.
Xiang, Y., H. Wen, Y. Yu, M. Li, X. Fu, and S. Huang. Gut-on-chip: recreating human intestine in vitro. J. Tissue Eng. 11:2041731420965318, 2020.
Xie, F., X. Ding, and Q.-Y. Zhang. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B. 6:374–383, 2016.
Zanger, U. M., and M. Schwab. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 138:103–141, 2013.
Zheng, L., C. J. Kelly, and S. P. Colgan. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309:C350–C360, 2015.
Zimmermann, M., M. Zimmermann-Kogadeeva, R. Wegmann, and A. L. Goodman. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 570:462–467, 2019.
Funding
This work was partly funded by the Nayar Prize II, Alternatives Research and Development Foundation (ARDF) and student scholarships from the Armor College of Engineering.
Author information
Authors and Affiliations
Contributions
Participated in research design: CW, AB. Conducted experiments: CW, AC, JB, DM, AAJ, AN. Contributed new reagents or analytic tools: CW, AB. Performed data analysis: CW, JB, AAJ, AB. Performed data visualization: CW, JB, AC, DM. Wrote or contributed to the writing of the manuscript: CW, AC, JB, DM, AB.
Corresponding author
Ethics declarations
Conflict of interest
The authors Chengyao Wang, Andrea Cancino, Jasmine Baste, Daniel Marten, Advait Anil Joshi, Amreen Nasreen, Abhinav Bhushan have declared that no conflict of interest exists.
Human Studies
No human studies were carried out by the authors for this article.
Animal Studies
No animal studies were carried out by the authors for this article.
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Cancino, A., Baste, J. et al. Causative Role of Anoxic Environment in Bacterial Regulation of Human Intestinal Function. Cel. Mol. Bioeng. 15, 493–504 (2022). https://doi.org/10.1007/s12195-022-00735-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00735-x