Abstract
Introduction
Recent studies have revealed that several deubiquitinating enzymes (DUBs) play important roles in hepatocellular carcinoma (HCC) progression, but the roles of Otubain 2 (OTUB2) in HCC remain obscure.
Methods
In this study, we investigated the expression of OTUB2 in HCC based on clinical samples and a public online database (ENCORI), and its roles and working mechanisms were further explored by in vitro experiments.
Results
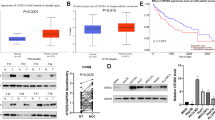
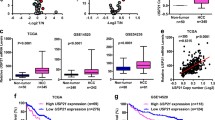
It was found that the expression of OTUB2 was significantly up-regulated in HCC tissues, and correlated with poor prognosis of HCC patients. Functionally, the overexpression of OTUB2 could promote malignant proliferation and metastasis of HCC cells, while knockdown of OTUB2 exerted the opposite results. Using two bioinformatics tools, PJA1 was identified as a potential gene regulated by OTUB2. Mechanistically, it was found that OTUB2 promoted the stabilization of PJA1 by deubiquitylation, based on immunoprecipitation (IP) and cycloheximide (CHX) assays. Moreover, the suppressive effects of OTUB2 depletion on the malignant phenotypes of HCC cells could be reversed by overexpressing PJA1.
Conclusion
In conclusion, our study indicated that OTUB2 could promote the malignant proliferation and migration of HCC cells by increasing the stability of PJA1 via deubiquitylation.






Similar content being viewed by others
References
Antao, A. M., A. Tyagi, K. S. Kim, and S. Ramakrishna. Advances in deubiquitinating enzyme inhibition and applications in cancer therapeutics. Cancers (Basel). 12:1579, 2020.
Chan, D. W., C. Y. Chan, J. W. Yam, Y. P. Ching, and I. O. Ng. Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 131:1218–1227, 2006.
Chen, J., and J. A. Gingold. Dysregulated PJA1-TGF-β signaling in cancer stem cell-associated liver cancers. Oncoscience. 7:88–95, 2020.
Chen, J., A. Mitra, S. Li, S. Song, B. N. Nguyen, J. S. Chen, J. H. Shin, N. R. Gough, P. Lin, V. Obias, A. R. He, Z. Yao, T. M. Malta, H. Noushmehr, P. S. Latham, X. Su, A. Rashid, B. Mishra, R. C. Wu, and L. Mishra. Targeting the E3 ubiquitin ligase PJA1 enhances tumor-suppressing TGFβ signaling. Cancer Res. 80:1819–1832, 2020.
Clague, M. J., C. Heride, and S. Urbé. The demographics of the ubiquitin system. Trends Cell Biol. 25:417–426, 2015.
Cockram, P. E., M. Kist, S. Prakash, S. H. Chen, I. E. Wertz, and D. Vucic. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 28:591–605, 2021.
Consalvi, S., A. Brancaccio, A. Dall’Agnese, P. L. Puri, and D. Palacios. Praja1 E3 ubiquitin ligase promotes skeletal myogenesis through degradation of EZH2 upon p38α activation. Nat. Commun. 8:13956, 2017.
Dey, A., D. Seshasayee, R. Noubade, D. M. French, J. Liu, M. S. Chaurushiya, D. S. Kirkpatrick, V. C. Pham, J. R. Lill, C. E. Bakalarski, J. Wu, L. Phu, P. Katavolos, L. M. LaFave, O. Abdel-Wahab, Z. Modrusan, S. Seshagiri, K. Dong, Z. Lin, M. Balazs, R. Suriben, K. Newton, S. Hymowitz, G. Garcia-Manero, F. Martin, R. L. Levine, and V. M. Dixit. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 337:1541–1546, 2012.
Fraile, J. M., V. Quesada, D. Rodríguez, J. M. Freije, and C. López-Otín. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 31:2373–2388, 2012.
Franke, F. C., J. Müller, M. Abal, E. D. Medina, U. Nitsche, H. Weidmann, S. Chardonnet, E. Ninio, and K. P. Janssen. The tumor suppressor SASH1 interacts with the signal adaptor CRKL to inhibit epithelial–mesenchymal transition and metastasis in colorectal cancer. Cell Mol. Gastroenterol. Hepatol. 7:33–53, 2019.
Gallo, L. H., J. Ko, and D. J. Donoghue. The importance of regulatory ubiquitination in cancer and metastasis. Cell Cycle. 16:634–648, 2017.
Goldberg, A. L. Protein degradation and protection against misfolded or damaged proteins. Nature. 426:895–899, 2003.
Gu, Z. L., J. Huang, and L. L. Zhen. Knockdown of otubain 2 inhibits liver cancer cell growth by suppressing NF-κB signaling. Kaohsiung J. Med. Sci. 36:399–404, 2020.
Haq, S., S. Das, D. H. Kim, A. P. Chandrasekaran, S. H. Hong, K. S. Kim, and S. Ramakrishna. The stability and oncogenic function of LIN28A are regulated by USP28. Biochim Biophys. Acta Mol. Basis Dis. 1865:599–610, 2019.
Harrigan, J. A., X. Jacq, N. M. Martin, and S. P. Jackson. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat. Rev. Drug Discov. 17:57–78, 2018.
Hussain, S., Y. Zhang, and P. J. Galardy. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 8:1688–1697, 2009.
Johnson, R., and G. Halder. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13:63–79, 2014.
Kuo, K. L., S. H. Liu, W. C. Lin, P. M. Chow, Y. W. Chang, S. P. Yang, C. S. Shi, C. H. Hsu, S. M. Liao, H. C. Chang, and K. H. Huang. The deubiquitinating enzyme inhibitor PR-619 enhances the cytotoxicity of cisplatin via the suppression of anti-apoptotic Bcl-2 protein: in vitro and in vivo study. Cells. 8:1268, 2019.
Li, Z., Z. Cheng, C. Raghothama, Z. Cui, K. Liu, X. Li, C. Jiang, W. Jiang, M. Tan, X. Ni, A. Pandey, J. O. Liu, and Y. Dang. USP9X controls translation efficiency via deubiquitination of eukaryotic translation initiation factor 4A1. Nucleic Acids Res. 46:823–839, 2018.
Li, J., D. Cheng, M. Zhu, H. Yu, Z. Pan, L. Liu, Q. Geng, H. Pan, M. Yan, and M. Yao. OTUB2 stabilizes U2AF2 to promote the Warburg effect and tumorigenesis via the AKT/mTOR signaling pathway in non-small cell lung cancer. Theranostics. 9:179–195, 2019.
Li, J. H., S. Liu, H. Zhou, L. H. Qu, and J. H. Yang. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 42:92–97, 2014.
Linares, J. F., A. Duran, T. Yajima, M. Pasparakis, J. Moscat, and M. T. Diaz-Meco. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell. 51:283–296, 2013.
Luo, Z., X. Ye, Y. Cheng, F. Li, F. Shou, and G. Wang. E3 ubiquitin ligase PJA1 regulates lung adenocarcinoma apoptosis and invasion through promoting FOXR2 degradation. Biochem. Biophys. Res. Commun. 556:106–113, 2021.
Nanao, M. H., S. O. Tcherniuk, J. Chroboczek, O. Dideberg, A. Dessen, and M. Y. Balakirev. Crystal structure of human otubain 2. EMBO Rep. 5:783–788, 2004.
Ohshiro, K., J. Chen, J. Srivastav, L. Mishra, and B. Mishra. Alterations in TGF-β signaling leads to high HMGA2 levels potentially through modulation of PJA1/SMAD3 in HCC cells. Genes Cancer. 11:43–52, 2020.
Padua, D., and J. Massagué. Roles of TGFbeta in metastasis. Cell Res. 19:89–102, 2009.
Pan, B., Y. Yang, J. Li, Y. Wang, C. Fang, F. X. Yu, and Y. Xu. USP47-mediated deubiquitination and stabilization of YAP contributes to the progression of colorectal cancer. Protein Cell. 11:138–143, 2020.
Pinto-Fernandez, A., and B. M. Kessler. DUBbing cancer: deubiquitylating enzymes involved in epigenetics, DNA damage and the cell cycle as therapeutic targets. Front. Genet. 7:133, 2016.
Rape, M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 19:59–70, 2018.
Rhodes, D. R., J. Yu, K. Shanker, N. Deshpande, R. Varambally, D. Ghosh, T. Barrette, A. Pandey, and A. M. Chinnaiyan. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 6:1–6, 2004.
Sasaki, A., Y. Masuda, K. Iwai, K. Ikeda, and K. Watanabe. A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J. Biol. Chem. 277:22541–22546, 2002.
Siegel, R. L., K. D. Miller, H. E. Fuchs, and A. Jemal. Cancer statistics, 2021. CA Cancer J. Clin. 71:7–33, 2021.
Sivakumar, D., V. Kumar, M. Naumann, and M. Stein. Activation and selectivity of OTUB-1 and OTUB-2 deubiquitinylases. J. Biol. Chem. 295:6972–6982, 2020.
Szklarczyk, D., A. L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-Cepas, M. Simonovic, N. T. Doncheva, J. H. Morris, P. Bork, L. J. Jensen, and C. V. Mering. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47:D607-d613, 2019.
Tang, Z., C. Li, B. Kang, G. Gao, C. Li, and Z. Zhang. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45:W98-w102, 2017.
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380:1450–1462, 2019.
Warde-Farley, D., S. L. Donaldson, O. Comes, K. Zuberi, R. Badrawi, P. Chao, M. Franz, C. Grouios, F. Kazi, C. T. Lopes, A. Maitland, S. Mostafavi, J. Montojo, Q. Shao, G. Wright, G. D. Bader, and Q. Morris. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 38:W214-220, 2010.
Zhang, Z., J. Du, S. Wang, L. Shao, K. Jin, F. Li, B. Wei, W. Ding, P. Fu, H. van Dam, A. Wang, J. Jin, C. Ding, B. Yang, M. Zheng, X. H. Feng, K. L. Guan, and L. Zhang. OTUB2 promotes cancer metastasis via hippo-independent activation of YAP and TAZ. Mol. Cell. 73:7-21.e27, 2019.
Acknowledgments
None.
Funding
No funding was received.
Conflict of interest
Gang Hu, Jianwu Yang, Hongwen Zhang, Zhen Huang and Heming Yang declare that they have no conflicts of interest.
Ethical Approval
The procedure of this research was reviewed and approved by Strategic Support Force Characteristic Medical Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Partha Roy oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, G., Yang, J., Zhang, H. et al. OTUB2 Promotes Proliferation and Migration of Hepatocellular Carcinoma Cells by PJA1 Deubiquitylation. Cel. Mol. Bioeng. 15, 281–292 (2022). https://doi.org/10.1007/s12195-022-00720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00720-4