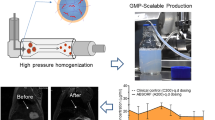
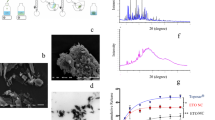
Abstract
Introduction
A major challenge in cancer medicine is the safe and effective delivery of drugs to the right tissue at the right time. Despite being designed for greater target specificity, many drugs still result in side effects and lack of safety in patients following global dissemination. Therefore, to develop new, more effective formulations capable of improving specificity and reducing off-target effects, here we describe formulation of drug crystals, from even a very hydrophobic and otherwise difficult to solubilize small molecule chemical compound, capable of providing constant drug release for weeks following a single injection.
Methods
We chose to utilize the multi-tyrosine kinase inhibitor and multi-modal (anti-angiogenic and tumor cell cytotoxic) agent sorafenib, to combat aberrant angiogenesis and tumor growth which contribute to metastasis, ultimately responsible for poor patient outcomes. We tuned crystal size (surface area:volume ratios), imaged by SEM, to display controllability of drug delivery kinetics in in vitro drug release assays.
Results
Single and powder crystal X-ray diffraction (XRD) established that all crystals were the same polymorph and drug form. When utilized against an orthotopic triple negative breast cancer (TNBC) mouse model (4T1 in syngeneic BALB/c mice), we established anti-tumor activity from a single local, subcutaneous injection of crystalline sorafenib.
Conclusion
From our findings, we support that engineering crystalline drug delivery systems has implications in the treatment of cancer or other diseases where high enough constitutive drug levels are needed to maintain target saturation and inhibition while also preventing emergence of drug resistance, which is a consequence often seen with suboptimal dosing.




Similar content being viewed by others
References
Baklaushev, V. P., A. Kilpeläinen, S. Petkov, M. A. Abakumov, and N. F. Grinenko. Luciferase expression allows bioluminescence imaging but imposes limitations on the orthotopic mouse (4T1) model of breast cancer. Nat. Sci. Rep. 2017. https://doi.org/10.1038/s41598-017-07851-z.
Barrett, P., et al. A review of the use of process analytical technology for the understanding and optimization of production batch crystallization processes abstract. Org. Process Res. Dev. 9:348–355, 2005. https://doi.org/10.1021/op049783p.
Bergers, G., and L. E. Benjamin. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003. https://doi.org/10.1038/nrc1093.
Bernstein, J. Polymorphism in Molecular Crystals 2e, 2nd ed. Oxford: Oxford University Press, 2020.
Bil, J., L. Zapala, D. Nowis, M. Jakobisiak, and J. Golab. Statins potentiate cytostatic / cytotoxic activity of sorafenib but not sunitinib against tumor cell lines in vitro. Cancer Lett. 288:57–67, 2010. https://doi.org/10.1016/j.canlet.2009.06.022.
Chen, J., B. Sarma, J. M. B. Evans, and A. S. Myerson. Pharmaceutical crystallization. Crystal Growth Des. 11:887–895, 2011. https://doi.org/10.1021/cg101556s.
Chin, S. Y., et al. Additive manufacturing of hydrogel-based materials for next-generation implantable medical devices. Sci. Robot. 2017. https://doi.org/10.1126/scirobotics.aah6451.
Davey, R., and J. Garside. From Molecules to Crystallizers: An Introduction to Crystallization. Oxford: Oxford University Press, 2000.
Devita, V. T., and E. Chu. A history of cancer chemotherapy. Cancer Res. 68:8643–8654, 2008. https://doi.org/10.1158/0008-5472.CAN-07-6611.
Fantozzi, A., and G. Christofori. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006. https://doi.org/10.1186/bcr1530.
Farah, S., et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat. Mater. 2019. https://doi.org/10.1038/s41563-019-0377-5.
Ferguson, F. M., and N. S. Gray. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 17:353–376, 2018. https://doi.org/10.1038/nrd.2018.21.
Genck, W. Better growth in batch crystallizers. Chem. Eng. 107:90–95, 2000.
Goon, P. K. Y., G. Y. H. Lip, P. S. Stonelake, and A. D. Blann. Circulating Endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the nottingham prognostic Index. Neoplasia. 11:771–779, 2009. https://doi.org/10.1593/neo.09490.
Grafone, T., M. Palmisano, C. Nicci, and S. Storti. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncol. Rev. 2012. https://doi.org/10.4081/oncol.2012.e8.
Hanahan, D., and R. A. Weinberg. Hallmarks of cancer: the next generation. Cell. 144:646–674, 2011. https://doi.org/10.1016/j.cell.2011.02.013.
Holderfield, M., M. M. Deuker, F. McCormick, and M. McMahon. Targeting RAF kinases for cancer therapy: BRAF mutated melanoma and beyond. Nature Reviews Cancer. 14:455–467, 2015. https://doi.org/10.1038/nrc3760.
Khan, W., S. Farah, A. Nyska, and A. J. Domb. Carrier free rapamycin loaded drug eluting stent : In vitro and in vivo evaluation. J. Controlled Release. 168:70–76, 2013. https://doi.org/10.1016/j.jconrel.2013.02.012.
Langley, R. R., and I. J. Fidler. The seed and soil hypothesis revisited - the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer. 128:2527–2535, 2012. https://doi.org/10.1002/ijc.26031.
Leong, A., K. Cooper, and J. Leong. Manual of Diagnostic Antibodies for Immunohistology, 2nd ed. London: Greenwich Medical Media, 2002.
Liu, Q., et al. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Molecular Cancer. 2017. https://doi.org/10.1186/s12943-017-0742-4.
Malekian, S., M. Rahmati, S. Sari, M. Kazemimanesh, and R. Kheirbakhsh. Expression of diverse angiogenesis factor in different stages of the 4T1 tumor as a mouse model of triple-negative breast cancer. Adv. Pharm. Bull. 10:323–328, 2020. https://doi.org/10.34172/apb.2020.039.
Murakami, H., Y. Ogata, Y. Akagi, N. Ishibashi, and K. Shirouzu. Circulating endothelial progenitor cells in metronomic chemotherapy using irinotecan and/or bevacizumab for colon carcinoma: study of their clinical significance. Exp. Ther. Med. 2:595–600, 2011. https://doi.org/10.3892/etm.2011.253.
Nittayacharn, P., and N. Nasongkla. Development of self-forming doxorubicin-loaded polymeric depots as an injectable drug delivery system for liver cancer chemotherapy. J. Mater. Sci. 2017. https://doi.org/10.1007/s10856-017-5905-8.
Olaparib – DrugBank. https://www.drugbank.ca/drugs/DB09074, 2020.
Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 8:98–101, 1889.
Psaila, B., R. N. Kaplan, E. R. Port, and D. Lyden. Priming the ‘soil’ for breast cancer metastasis: the pre-metastatic niche. Breast Dis. 26:65–74, 2007.
Puhl, S., L. Meinel, and O. Germershaus. Recent advances in crystalline and amorphous particulate protein formulations. Asian J. Pharm. Sci. 11:469–477, 2016. https://doi.org/10.1016/j.ajps.2016.06.003.
Ray, P., N. Krishnamoorthy, and A. Ray. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell Cycle. 7:2826–2832, 2008.
Ryden, L., K. Jirstrom, O. S. Haglund, and M. Ferno. Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res. Treat. 120:491–498, 2010. https://doi.org/10.1007/s10549-010-0758-6.
Shi, K., H. Bi, and Y. Jiang. ScienceDirect characterization of physiochemical and biological properties of spherical protein crystals for sustained release. Asian J. Pharm. Sci. 8:58–63, 2013. https://doi.org/10.1016/j.ajps.2013.07.007.
Triple-negative Breast Cancer. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/types-of-breast-cancer/triple-negative.html, 2019.
Wang, C., et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 10:1–13, 2018. https://doi.org/10.1126/scitranslmed.aan3682.
Weigelt, B., J. L. Peterse, and L. J. V. Veer. Breast cancer metastasis. Nat. Rev. Cancer. 5:591–603, 2005. https://doi.org/10.1038/nrc1670.
Wilhelm, S. M., et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64:7099–7109, 2004. https://doi.org/10.1158/0008-5472.CAN-04-1443.
Wilhelm, S. M., et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 7:3129–3140, 2008. https://doi.org/10.1158/1535-7163.MCT-08-0013.
Yao, H., et al. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 8:1913–1924, 2015. https://doi.org/10.18632/oncotarget.12284.
Zhang, C., et al. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials. 84:1–12, 2016. https://doi.org/10.1016/j.biomaterials.2016.01.027.
Acknowledgments
This work was supported by the Biomedical Engineering Department at Johns Hopkins University. S.Y.N. and A.R. were both supported by GRFP fellowships from the National Science Foundation. The authors would like to acknowledge the use of resources at Johns Hopkins University School of Medicine Core Facilities for Microscopy, SEM, Histology, and Whole Animal Imaging.
Author Contributions
V.L., S.Y.N., A.R., and J.C.D. designed experiments, analyzed data, and wrote the manuscript. V.L., S.Y.N., A.R., J.P., J.S., S.L., A.S., and J.C.D. performed experiments. V.L., S.Y.N., A.R., J.S., and J.C.D. performed statistical analyses of data sets and aided in the preparation of displays communicating data sets. J.C.D. supervised the study. All authors discussed the results and assisted in the preparation of the manuscript.
Conflict of interest
All authors (Victoria Lai, Sarah Y. Neshat, Amanda Rakoski, James Pitingolo, Johndavid Sabedra, Stephen Li, Aryaman Shodhan, and Joshua C. Doloff) declare they have no conflict of interest.
Ethical Approval
There were no human studies carried out by the authors for this article. For all animal studies, they were carried out in accordance with IACUC-approved protocols at the Johns Hopkins University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the 2021 CMBE Young Innovators special issue.
Joshua C. Doloff is a new Assistant Professor in Biomedical Engineering, Materials Science, and Oncology (Cancer Immunology) at Johns Hopkins University. At the Translational Tissue Engineering Center, his lab focuses on Immunoengineering and Regenerative Medicine, with emphasis on cancer, autoimmunity, and implant/transplantation. Josh earned his undergraduate Bioengineering degree at the University of Pennsylvania, where he carried out biomaterials and tissue engineering research in the Ducheyne Lab. Later, to better understand what happens when deliverables are introduced into the body, Joshua focused his PhD in the Waxman Lab at Boston University on host immune responses to varied therapeutics. Early work on cancer-targeted viral vectors won technology development and University Provost awards. Later work produced insights into chemotherapy-induced anti-tumor immunity. Highlighting his achievements, Joshua was awarded the Frank A. Belamarich Award for Best Doctoral Research in his graduating class. Josh went on to become a Juvenile Diabetes Research Foundation (JDRF) Postdoctoral Fellow in the Langer and Anderson labs at the Koch Institute at MIT. There, his work on deciphering immune-mediated biomaterial and biomedical device implant rejection contributed to numerous top publications, patents, a lab startup—Sigilon, and awards—including top presentation selections, Immunoengineering prizes, co-chair honors, and a Rising Star Alumni Award.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lai, V., Neshat, S.Y., Rakoski, A. et al. Crystallization of the Multi-Receptor Tyrosine Kinase Inhibitor Sorafenib for Controlled Long-Term Drug Delivery Following a Single Injection. Cel. Mol. Bioeng. 14, 471–486 (2021). https://doi.org/10.1007/s12195-021-00708-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00708-6