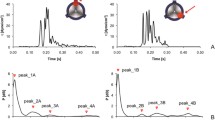
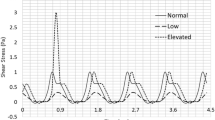
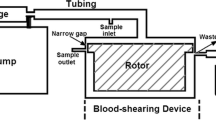
Abstract
Introduction
Platelet activation by mechanical means such as shear stress exposure, is a vital driver of thrombotic risk in implantable blood-contacting devices used in the treatment of heart failure. Lipids are essential in platelets activation and have been studied following biochemical activation. However, little is known regarding lipid alterations occurring with mechanical shear-mediated platelet activation.
Methods
Here, we determined if shear-activation of platelets induced lipidome changes that differ from those associated with biochemically-mediated platelet activation. We performed high-resolution lipidomic analysis on purified platelets from four healthy human donors. For each donor, we compared the lipidome of platelets that were non-activated or activated by shear, ADP, or thrombin treatment.
Results
We found that shear activation altered cell-associated lipids and led to the release of lipids into the extracellular environment. Shear-activated platelets released 21 phospholipids and sphingomyelins at levels statistically higher than platelets activated by biochemical stimulation.
Conclusions
We conclude that shear-mediated activation of platelets alters the basal platelet lipidome. Further, these alterations differ and are unique in comparison to the lipidome of biochemically activated platelets. Many of the released phospholipids contained an arachidonic acid tail or were phosphatidylserine lipids, which have known procoagulant properties. Our findings suggest that lipids released by shear-activated platelets may contribute to altered thrombosis in patients with implanted cardiovascular therapeutic devices.







Similar content being viewed by others
References
Akiba, S., T. Murata, K. Kitatani, and T. Sato. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol. Pharma Bull. 23:1293–1297, 2000.
Alkhamis, T. M., R. L. Beissinger, and J. R. Chediak. Artificial surface effect on red blood cells and platelets in laminar shear flow. Blood. 75:1568–1575, 1990.
Alves, M. A., S. Lamichhane, A. Dickens, A. McGlinchey, H. C. Ribeiro, P. Sen, et al. Systems biology approaches to study lipidomes in health and disease. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 1866(2):158857, 2020.
Apostoli, A., V. Bianchi, N. Bono, A. Dimasi, K. R. Ammann, Y. R. Moiia, et al. Prothrombotic activity of cytokine-activated endothelial cells and shear-activated platelets in the setting of ventricular assist device support. J. Heart Lung Transplant. 38:658–667, 2019.
Barry, O. P., D. Pratico, J. A. Lawson, and G. A. FitzGerald. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J. Clin. Invest. 99:2118–2127, 1997.
Benjamin, E. J., P. Muntner, A. Alonso, M. S. Bittencourt, C. W. Callaway, A. P. Carson, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 139:e56–e528, 2019.
Biró, E., J. W. Akkerman, F. J. Hoek, G. Gorter, L. M. Pronk, A. Sturk, et al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J. Thromb. Haemost. 3:2754–2763, 2005.
Bluestein, D., Y. M. Li, and I. B. Krukenkamp. Free emboli formation in the wake of bi-leaflet mechanical heart valves and the effects of implantation techniques. J. Biomech. 35:1533–1540, 2002.
Chang, C. P., J. Zhao, T. Wiedmer, and P. J. Sims. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J. Biol. Chem. 268:7171–7178, 1993.
Chen, Z., S. K. Jena, G. A. Giridharan, S. C. Koenig, M. S. Slaughter, B. P. Griffith, et al. Flow features and device-induced blood trauma in CF-VADs under a pulsatile blood flow condition: A CFD comparative study. Int. J. Numer. Method. Biomed. Eng. 34:15, 2018.
Chiu, W. C., P. L. Tran, Z. Khalpey, E. Lee, Y. R. Woo, M. J. Slepian, et al. Device thrombogenicity emulation: an in silico predictor of in vitro and in vivo ventricular assist device thrombogenicity. Sci. Rep. 9:2946, 2019.
Clark, S. R., C. P. Thomas, V. J. Hammond, M. Aldrovandi, G. W. Wilkinson, K. W. Hart, et al. Characterization of platelet aminophospholipid externalization reveals fatty acids as molecular determinants that regulate coagulation. Proc. Natl. Acad. Sci U. S. A. 110:5875–5880, 2013.
Fadeel, B., and D. Xue. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44:264–277, 2009.
Fahy, E., S. Subramaniam, R. C. Murphy, M. Nishijima, C. R. Raetz, T. Shimizu, et al. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid. Res. 50:S9–S14, 2009.
Foin, N., J. L. Gutierrez-Chico, S. Nakatani, R. Torii, C. V. Bourantas, S. Sen, et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ. Cardiovasc. Interv. 7:180–189, 2014.
Frelinger, A. L., 3rd., M. I. Furman, M. D. Linden, Y. Li, M. L. Fox, M. R. Barnard, et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation. 113:2888–2896, 2006.
Gao, Z., F. Liu, Z. Yu, X. Bai, C. Yang, F. Zhuang, et al. Effects of von Willebrand factor concentration and platelet collision on shear-induced platelet activation. Thromb. Haemost. 100:60–68, 2008.
Girdhar, G., M. Xenos, Y. Alemu, W. C. Chiu, B. E. Lynch, J. Jesty, et al. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS ONE. 7:32463, 2012.
Hanke, J. S., G. Dogan, L. Wert, M. Ricklefs, J. Heimeshoff, A. Chatterjee, et al. Left ventricular assist device exchange for the treatment of HeartMate II pump thrombosis. J. Thorac. Dis. 10:S1728–S1736, 2018.
Hansen, C. E., Y. Qiu, O. J. T. McCarty, and W. A. Lam. Platelet Mechanotransduction. Annu. Rev. Biomed. Eng. 20:253–275, 2018.
Heijnen, H. F. G., and S. J. A. Korporaal. Platelet Morphology and Ultrastructure. In: Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: an Update, edited by P. Gresele, N. S. Kleiman, J. A. Lopez, and C. P. Page. Cham: Springer International Publishing, 2017, pp. 21–37.
Hemker, H. C., J. L. van Rijn, J. Rosing, G. van Dieijen, E. M. Bevers, and R. F. Zwaal. Platelet membrane involvement in blood coagulation. Blood Cells. 9:303–317, 1983.
Holme, P. A., U. Orvim, M. J. Hamers, N. O. Solum, F. R. Brosstad, R. M. Barstad, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler. Thromb. Vasc. Biol. 17:646–653, 1997.
Hu, Q., M. Wang, M. S. Cho, C. Wang, A. M. Nick, P. Thiagarajan, et al. Lipid profile of platelets and platelet-derived microparticles in ovarian cancer. BBA Clin. 6:76–81, 2016.
Jakubowski, J. A., and N. G. Ardlie. Evidence for the mechanism by which eicosapentaenoic acid inhibits human platelet aggregation and secretion - implications for the prevention of vascular disease. Thromb. Res. 16:205–217, 1979.
Jesty, J., and D. Bluestein. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal. Biochem. 272:64–70, 1999.
Jesty, J., W. Yin, P. Perrotta, and D. Bluestein. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 14:143–149, 2003.
Kapil, N., Y. H. Datta, N. Alakbarova, E. Bershad, M. Selim, D. S. Liebeskind, et al. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin. Appl. Thromb. Hemost. 23:301–318, 2017.
Kasahara, K., M. Kaneda, T. Miki, K. Iida, N. Sekino-Suzuki, I. Kawashima, et al. Clot retraction is mediated by factor XIII-dependent fibrin-alphaIIbbeta3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood. 122:3340–3348, 2013.
Kheradvar, A., and G. Pedrizzetti. Vortex Formation in the Cardiovascular System. New York: Springer, 2012.
Kirklin, J. K., D. C. Naftel, R. L. Kormos, F. D. Pagani, S. L. Myers, L. W. Stevenson, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J. Heart Lung Transpl. 33:12–22, 2014.
Koseoglu, S., A. F. Meyer, D. Kim, B. M. Meyer, Y. Wang, J. J. Dalluge, et al. Analytical characterization of the role of phospholipids in platelet adhesion and secretion. Anal. Chem. 87:413–421, 2015.
LaDisa, J. F., L. E. Olson, I. Guler, D. A. Hettrick, S. H. Audi, J. R. Kersten, et al. Stent design properties and deployment ratio influence indexes of wall shear stress: a three-dimensional computational fluid dynamics investigation within a normal artery. J. Appl. Physiol. 2004(97):424–430, 1985.
Lepropre, S., S. Kautbally, M. Octave, A. Ginion, M. B. Onselaer, G. R. Steinberg, et al. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood. 132:1180–1192, 2018.
Leung, S. L., Y. Lu, D. Bluestein, and M. J. Slepian. Dielectrophoresis-mediated electrodeformation as a means of determining individual platelet stiffness. Ann. Biomed. Eng. 44:903–913, 2016.
Lhermusier, T., H. Chap, and B. Payrastre. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 9:1883–1891, 2011.
Lim, W. Y., G. Lloyd, and S. Bhattacharyya. Mechanical and surgical bioprosthetic valve thrombosis. Heart. 103:1934–1941, 2017.
Marcus, A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J. Lipid. Res. 19:793–826, 1978.
Melamud, E., L. Vastag, and J. D. Rabinowitz. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 82:9818–9826, 2010.
Michelson, A. D., M. Cattaneo, J. W. Eikelboom, P. Gurbel, K. Kottke-Marchant, T. J. Kunicki, et al. Aspirin resistance: position paper of the Working Group on Aspirin Resistance. J. Thromb. Haemost. 3:1309–1311, 2005.
Miyazaki, Y., S. Nomura, T. Miyake, H. Kagawa, C. Kitada, H. Taniguchi, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 88:3456–3464, 1996.
Nobili, M., J. Sheriff, U. Morbiducci, A. Redaelli, and D. Bluestein. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 54:64–72, 2008.
O’Donnell, V. B., R. C. Murphy, and S. P. Watson. Platelet lipidomics: modern day perspective on lipid discovery and characterization in platelets. Circ.Res. 114:1185–1203, 2014.
Patrono, C., B. Coller, G. A. FitzGerald, J. Hirsh, and G. Roth. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP conference on Antithrombotic and Thrombolytic Therapy. Chest. 126:234S-264S, 2004.
Peng, B., S. Geue, C. Coman, P. Münzer, D. Kopczynski, C. Has, et al. Identification of key lipids critical for platelet activation by comprehensive analysis of the platelet lipidome. Blood. 132:e1–e12, 2018.
Pienimaeki-Roemer, A., K. Kuhlmann, A. Bottcher, T. Konovalova, A. Black, E. Orso, et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion. 55:507–521, 2015.
Prescott, S. M., and P. W. Majerus. The fatty acid composition of phosphatidylinositol from thrombin-stimulated human platelets. J. Biol. Chem. 256:579–582, 1981.
Reejhsinghani, R., and A. S. Lotfi. Prevention of stent thrombosis: challenges and solutions. Vasc. Health Risk Manag. 11:93–106, 2015.
Roka-Moiia, Y., K. R. Ammann, S. Miller-Gutierrez, A. Sweedo, D. Palomares, J. Italiano, et al. Shear-mediated platelet activation in the free flow II: Evolving mechanobiological mechanisms reveal an identifiable signature of activation and a bi-directional platelet dyscrasia with thrombotic and bleeding features. J. Biomech. 123:110415, 2021.
Roka-Moiia, Y., S. Miller-Gutierrez, D. E. Palomares, J. E. Italiano, J. Sheriff, D. Bluestein, et al. Platelet dysfunction during mechanical circulatory support: elevated shear stress promotes downregulation of alphaIIbbeta3 and GPIb via microparticle shedding decreasing platelet aggregability. Arterioscler. Thromb. Vasc. Biol. 41:1319–1336, 2021.
Roka-Moiia, Y., R. Walk, D. E. Palomares, K. R. Ammann, A. Dimasi, J. E. Italiano, et al. Platelet activation via shear stress exposure induces a differing pattern of biomarkers of activation versus biochemical agonists. Thromb. Haemost. 120:776–792, 2020.
Sakariassen, K. S., P. A. Holme, U. Orvim, R. M. Barstad, N. O. Solum, and F. R. Brosstad. Shear-induced platelet activation and platelet microparticle formation in native human blood. Thromb. Res. 92:S33-41, 1998.
Sheriff, J., D. Bluestein, G. Girdhar, and J. Jesty. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann. Biomed. Eng. 38:1442–1450, 2010.
Sheriff, J., J. S. Soares, M. Xenos, J. Jesty, M. J. Slepian, and D. Bluestein. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann. Biomed. Eng. 41:1279–1296, 2013.
Sheriff, J., P. L. Tran, M. Hutchinson, T. DeCook, M. J. Slepian, D. Bluestein, et al. Repetitive hypershear activates and sensitizes platelets in a dose-dependent manner. Artif. Organs. 40:586–595, 2016.
Siess, W., P. Roth, B. Scherer, I. Kurzmann, B. Bohlig, and P. C. Weber. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1:441–444, 1980.
Slatter, D. A., M. Aldrovandi, A. O’Connor, S. M. Allen, C. J. Brasher, R. C. Murphy, et al. Mapping the human platelet lipidome reveals cytosolic phospholipase A2 as a regulator of mitochondrial bioenergetics during activation. Cell Metab. 23:930–944, 2016.
Slepian, M. J., J. Sheriff, M. Hutchinson, P. Tran, N. Bajaj, J. G. Garcia, et al. Shear-mediated platelet activation in the free flow: perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J. Biomech. 50:20–25, 2017.
Soares, J. S., C. Gao, Y. Alemu, M. Slepian, and D. Bluestein. Simulation of platelets suspension flowing through a stenosis model using a dissipative particle dynamics approach. Ann. Biomed. Eng. 41:2318–2333, 2013.
Starling, R. C., N. Moazami, S. C. Silvestry, G. Ewald, J. G. Rogers, C. A. Milano, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. New EnglJ. Med. 370:33–40, 2014.
Sweedo, A., M. Niemiec, L. Breshears, J. Sherriff, D. Bluestein, M. J. Slepian, et al. Repetitive Mechanostimulation of Platelets Alters Regional Membrane Stiffness and Its Distribution. Chicago IL: American Society for Artificial Internal Organs, 2020.
Sweedo, A., Y. Roka-Moiia, J. Sheriff, D. Bluestein, F. T. Arce, and M. J. Slepian. Shear-Mediated Platelet Activation is Associated with Global and Local Changes in Biomechanical Properties: Implications for Mechanism and Therapy. San Francisco CA: American Society for Artificial Internal Organs, 2019.
Sweedo, A., S. S. Saavedra, J. Sheriff, D. Bluestein, and M. J. Slepian. Platelet Membrane Fluidity: A Mechanistic Component of Shear-Mediated Platelet Activation. Washington DC: American Society for Artificial Internal Organs, 2018.
Tavoosi, N., R. L. Davis-Harrison, T. V. Pogorelov, Y. Z. Ohkubo, M. J. Arcario, M. C. Clay, et al. Molecular determinants of phospholipid synergy in blood clotting. J. Biol. Chem. 286:23247–23253, 2011.
Thomas, S. G. The Structure of Resting and Activated Platelets. In: Platelets, edited by A. D. Michelson. Cambridge, MA: Academic Press, 2019, pp. 47–77.
Tran, P. L., L. Valerio, J. Yamaguchi, W. Brengle, T. DeCook, M. Hutchinson, et al. Dimethyl Sulfoxide: A New Nemesis of Shear-Induced Platelet Activation. San Francisco, CA: Nanoengineering for Medicine and Biology, 2014.
Tsigkou, V., G. Siasos, K. Rovos, N. Tripyla, and D. Tousoulis. Peripheral artery disease and antiplatelet treatment. Curr. Opin. Pharmacol. 39:43–52, 2018.
Valerio, L., J. Sheriff, P. L. Tran, W. Brengle, A. Redaelli, G. B. Fiore, et al. Routine clinical anti-platelet agents have limited efficacy in modulating hypershear-mediated platelet activation associated with mechanical circulatory support. Thromb. Res. 163:162–171, 2018.
Valerio, L., P. L. Tran, J. Sheriff, W. Brengle, R. Ghosh, W. C. Chiu, et al. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thromb. Res. 140:110–117, 2016.
Viisoreanu, D., and A. Gear. Effect of physiologic shear stresses and calcium on agonist-induced platelet aggregation, secretion, and thromboxane A2 formation. Thromb. Res. 120:885–892, 2007.
Virani, S. S., A. Alonso, E. J. Benjamin, M. S. Bittencourt, C. W. Callaway, A. P. Carson, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 141:e139–e596, 2020.
Wei, R., J. Wang, M. Su, E. Jia, S. Chen, T. Chen, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci. Rep. 8:663, 2018.
Wolberg, A. S., F. R. Rosendaal, J. I. Weitz, I. H. Jaffer, G. Agnelli, T. Baglin, et al. Venous thrombosis. Nat. Rev. Dis. Primers. 1:15006, 2015.
Xi, Y., S. Harwood, L. Wise, and J. G. Purdy. Human cytomegalovirus pUL37x1 is important to remodeling of host lipid metabolism. J. Virol. 93:1–19, 2019.
Xi, Y., L. Lindenmayer, I. Kline, J. von Einem, and J. G. Purdy. Human cytomegalovirus uses a host stress response to balance the elongation of saturated/monounsaturated and polyunsaturated very-long-chain fatty acids. mBio. 12:48, 2021.
Yun, S. H., E. H. Sim, R. Y. Goh, J. I. Park, and J. Y. Han. Platelet activation: the mechanisms and potential biomarkers. Biomed. Res. Int. 2016:9060143, 2016.
Zhang, P., C. Gao, N. Zhang, M. J. Slepian, Y. Deng, and D. Bluestein. Multiscale particle-based modeling of flowing platelets in blood plasma using dissipative particle dynamics and coarse grained molecular dynamics. Cell Mol. Bioeng. 7:552–574, 2014.
Acknowledgments
We thank Debbie Mustacich for assistance in the initial stages of this project. We also thank the University of Arizona BIO5 Institute Statistics Consulting Lab for helpful discussion. This work was supported by the Arizona Biomedical Research Commission through the Arizona Department of Health Services (ADHS18-198868 to J.G.P.), the National Institute of Health (NIH) (T32HL007955 to A.S. and 5U01HL131052 to D.B. and M.J.S.), BIO5 Institute at the University of Arizona (Pilot Interdisciplinary Project to J.G.P, S.S.S. and M.J.S.). The content is solely the responsibility of the authors and does not necessarily represent the views of ADHS and NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Alice Sweedo, Lisa M. Wise, Yana Roka-Moiia, Fernando Teran Arce, S. Scott Saavedra, Jawaad Sheriff, Danny Bluestein, Marvin J. Slepian, and John G. Purdy declare that they have no conflicts of interest.
Ethical approval
All human subjects research was carried out in accordance with the University of Arizona Institutional Review Board (IRB) approved protocol (University of Arizona IRB #1810013264). Informed consent was obtained from all donors included in the study. No animal studies were carried out by the authors for this article.
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12195_2021_692_MOESM1_ESM.tif
Platelet lipidomes from individual donors. The number of lipids identified by MS1 analysis are shown for each class of lipids. Supplementary file1 (TIF 444 kb)
12195_2021_692_MOESM2_ESM.tif
Principal component analysis (PCA) of lipids found in all four donors. (a) PCA analysis was performed in Origin Pro for lipids identified in the released and cell-associated fractions in all four donors. Included were Quality Control (QC) samples of lipids extracted from pooled human serum reference material from the National Institute of Standards and Technology (NIST). (b) PCA of lipids found in the released fraction of all four donors. (c) PCA of lipids found in the cell-associated fraction of all four donors. Supplementary file2 (TIF 726 kb)
12195_2021_692_MOESM3_ESM.tif
Relative fold-change of lipids found in all four donors. The heatmap shows the relative levels of lipids measured in the released fraction of all four donors. The abundance of each lipid observed in the activated-platelet relative to its abundance in the non-activated platelet is shown in color on a log2-scale. The heatmap was hierarchical clustered using Ward's method implemented using the Seaborn library of python. Modes of activation are represented by colored boxes above each column (HSD is represented by red boxes, Thrombin by purple boxes, and ADP by black boxes). The four individual donors are listed at the bottom of the columns. Supplementary file3 (TIF 1023 kb)
12195_2021_692_MOESM4_ESM.tif
Lipids significantly altered by shear activation in three or more donors. Lipids found in the composite platelet lipidome shown in Figure 2B were tested for >2-fold change in relative lipid level and statistical significance (p ≤ 0.05). The levels of the lipid in activated platelets relative to non-activated plates is shown. Lipids significantly downregulated are shown in blue and those upregulated are show in red. Supplementary file4 (TIF 459 kb)
12195_2021_692_MOESM5_ESM.tif
Some lipid levels are significantly different between HSD-treated shear activation and ADP-treated biochemical activation in the released fraction. Analysis and visualization were done as described in Figure 5. (a) Analysis of lipids in the released fraction. (b) Analysis of lipids in the cell-associated fraction. Supplementary file5 (TIF 826 kb)
Rights and permissions
About this article
Cite this article
Sweedo, A., Wise, L.M., Roka-Moiia, Y. et al. Shear-Mediated Platelet Activation is Accompanied by Unique Alterations in Platelet Release of Lipids. Cel. Mol. Bioeng. 14, 597–612 (2021). https://doi.org/10.1007/s12195-021-00692-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00692-x