Abstract
Introduction
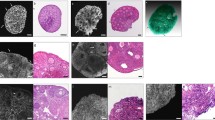
The use of 3D-bioprinted ovaries has been proven to be a promising technique for preserving fertility. Stereology is an accurate method to obtain quantitative 3D information and the stereological data is the basis for 3D bioprinting ovaries.
Methods
In this study, six female mice were used to acquire the ovarian tissues. One of the two paraffin-embedded ovaries of each mouse was cut into 5 µm sections, and the other was cut into 15 µm sections and then subjected to haematoxylin and eosin staining and anti-follicle stimulating hormone receptor antibody immunohistochemistry. The volume and volume fractions of ovaries were measured by the Cavalieri method. Then, the numerical densities and total numbers of ovarian granulosa cells (OGCs) and primordial, preantral and antral follicles in serial sections were estimated using design-based stereology.
Results
The ovarian volume was 2.50 ± 0.32 mm3. The volume fractions of the cortex, medulla, follicles and OGCs were 86.80% ± 2.82, 13.20% ± 2.82%, 5.60% ± 0.25% and 81.19% ± 2.57%, respectively. The numerical densities of OGCs, the primordial, preantral and antral follicles were 2.11 (± 0.28) × 106/mm3, 719.57 ± 18.04/mm3, 71.84 ± 3.93/mm3 and 17.29 ± 3.54/mm3, respectively. The total number of OGCs and follicles per paraffin-embedded ovary were 5.26 (± 0.09) × 106 and 2013.66 ± 8.16.
Conclusions
The study had obtained the stereological data of the mice ovaries, which contribute to a deeper understanding of the structure of the ovaries. Meanwhile, the data will supply information for 3D bioprinting ovaries.




Similar content being viewed by others
References
Chiti, M. C., M. M. Dolmans, C. M. Lucci, et al. Further insights into the impact of mouse follicle stage on graft outcome in an artificial ovary environment. Mol. Hum. Reprod. 23:381–392, 2017.
Codacci-Pisanelli, G., L. Del Pup, M. Del Grande, et al. Mechanisms of chemotherapy-induced ovarian damage in breast cancer patients. Crit. Rev. Oncol. Hematol. 113:90–96, 2017.
Dolmans, M. M., V. Luyckx, J. Donnez, C. Y. Andersen, and T. Greve. Affiliations expand risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 99:1514–1522, 2013.
Fisch, B., and R. Abir. Female fertility preservation: past, present and future. Reproduction 156:F11–F27, 2018.
Grigoryan, B., S. J. Paulsen, D. C. Corbett, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364:458–464, 2019.
Gundersen, H. J., T. F. Bendtsen, L. Korbo, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394, 1988.
Howell, K., N. Hopkins, and P. Mcloughlin. Combined confocal microscopy and stereology: a highly efficient and unbiased approach to quantitative structural measurement in tissues. Exp. Physiol. 87:747–756, 2002.
Jafarinezhad, Z., A. Rafati, F. Ketabchi, et al. Cardioprotective effects of curcumin and carvacrol in doxorubicin-treated rats: Stereological study. Food Sci. Nutr. 7:3581–3588, 2019.
Kang, H. W., S. J. Lee, I. K. Ko, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34:312–319, 2016.
Kubíková, T., P. Kochová, P. Tomášek, et al. Numerical and length densities of microvessels in the human brain: Correlation with preferential orientation of microvessels in the cerebral cortex, subcortical grey matter and white matter, pons and cerebellum. J. Chem. Neuroanat. 88:22–32, 2018.
Laronda, M. M., A. L. Rutz, S. Xiao, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 8:15261, 2017.
Liu, T., Y. Huang, J. Zhang, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 23:1548–1557, 2014.
Liu, T., W. Qin, Y. Huang, et al. Induction of estrogen-sensitive epithelial cells derived from human-induced pluripotent stem cells to repair ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. DNA Cell Biol. 32:685–698, 2013.
Liu, W., Q. Xin, X. Wang, et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 8:2017.
Osaki, T., S. G. M. Uzel, and R. D. Kamm. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 4:eaat5847, 2018.
Paraš, S., O. Janković, D. Trišić, et al. Influence of nanostructured calcium aluminate and calcium silicate on the liver: histological and unbiased stereological analysis. Int. Endod. J. 52:1162–1172, 2019.
Peng, B., J. Y. Lin, Y. Shang, et al. Plasticity in the synaptic number associated with neuropathic pain in the rat spinal dorsal horn: a stereological study. Neurosci. Lett. 486:24–28, 2010.
Revel, A., N. Laufer, A. Ben Meir, et al. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum. Reprod. 26:1097–1103, 2011.
Salama, M., and T. K. Woodruff. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet. Gynecol. Scand. 98:659–664, 2019.
Shojafar, E., M. Soleimani Mehranjani, and S. M. A. Shariatzadeh. Adipose-derived mesenchymal stromal cell transplantation at the graft site improves the structure and function of autografted mice ovaries: a stereological and biochemical analysis. Cytotherapy 20:1324–1336, 2018.
Siegel, R. L., K. D. Miller, and A. Jemal. Cancer statistics, 2018. CA Cancer J. Clin. 68:7–30, 2018.
Sterio, D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 134:127–136, 1984.
Yang, Z. W. Essential Tools for Morphometric Studies of Biological Tissues: Practical Stereological Methods. Bejing: Cornell University Press, p. 97, 2012.
Author contributions
XHH, ZWY and JHZ had designed the research. JHZ drafted the manuscript. JHZ, JKZ, YPT and YBS collected all the data. JHZ, JKZ and YPT analyzed the data and made all the figures in this manuscript. XHH had guided the writing. All authors read and approved the final manuscript.
Funding
Not applicable.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
This study was approved by the Animal Care and Use Committee of the Second Hospital of Hebei Medical University.
Consent to Participate
All animal-handling procedures were carried out according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication no. 85-23, revised 1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kaiming Ye oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, JH., Zhang, JK., Tian, YP. et al. A Stereological Study of Mouse Ovary Tissues for 3D Bioprinting Application. Cel. Mol. Bioeng. 14, 259–265 (2021). https://doi.org/10.1007/s12195-021-00668-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00668-x