Abstract
Introduction
The applied heat level and expose time are main issues in certain operations/applications, such as a laser assisted tissue welding, preparation of collagen-based biomaterials (films, implants). Therefore, the precise investigation of these parameters is crucial. The results can serve as a guideline to assess potential effects while maintaining the functionality of the collagen structures.
Methods
Collagen tissues from rat-tail tendon, calfskin, and bones are soaked in buffer solutions, then examined by microscope at different temperature levels.
Results
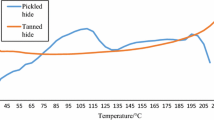
Increase in temperature reduced the microscopically observed collagen crimp contrast for calfskin and rat-tail tendons but not for bone tissues. The contrast level for rat tail tendon decreased down to 80% of its initial value at 37, 157, and 266 s for 70, 65, and 60 °C, respectively. The decrease in the crimp contrast was about only 25% and 2% at 55 and 50 °C after 2 h, respectively. 50% drop in contrast level was occurred for the skin samples at 16, 90, 110 and 1900 s for 70, 65, and 60 °C, respectively. The bone samples, did not show any significant differences in contrast levels.
Conclusion
The observed denaturation behaviours are in line with Arrhenius Law. This study could be expanded on to other types of tissues at wider temperature ranges to make a guideline for biological/medical processes that radiate heat in order to assess their side effects on collagen and other proteins.






Similar content being viewed by others
References
Bhagavan, N. V., and C.-E. Ha. Essentials of Medical Biochemistry: With Clinical Cases (2nd ed.). Amsterdam: Elsevier, 2015.
Bosch, T., A. Manich, J. Carilla, J. Cot, A. Marsal, H.-J. Kellert, et al. Collagen thermal transitions in chrome leather—thermogravimetry and differential scanning calorimetry. J. Am. Leather Chem. Assoc. 97:441–450, 2002.
Bürck, J., O. Aras, L. Bertinetti, C. A. Ilhan, M. A. Ermeydan, R. Schneider, A. S. Ulrich, and M. Kazanci. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 1151:73–80, 2018.
Bürck, J., S. Heissler, U. Geckle, M. F. Ardakani, R. Schneider, A. S. Ulrich, and M. Kazanci. Resemblance of electrospun collagen nanofibers to their native structure. Langmuir 29:1562–1572, 2013.
Cadenaro, M., L. Fontanive, C. O. Navarra, P. Gobbi, A. Mazzoni, R. Di Lenarda, et al. Effect of carboidiimide on thermal denaturation temperature of dentin collagen. Dent. Mater. 32:492–498, 2016.
Çakir, H., S. Genç, and E. Güler. 360-degree Iris burns following conductive keratoplasty. J. Refract. Surg. 32(11):776–778, 2016.
Chae, Y., G. Aguilar, E. J. Lavernia, and B. J. F. Wong. Characterization of temperature dependent mechanical behavior of cartilage. Lasers Surg. Med. 32:271–278, 2003.
De Boer, J., S. Srinivas, A. Malekafzali, Z. Chen, and J. Nelson. Imaging thermally damaged tissue by polarization sensitive optical coherence tomography. Opt. Express 3:212–218, 1998.
Eleswarapu, S. V., D. J. Responte, and K. A. Athanasiou. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS ONE 6:e26178, 2011.
Fitzpatrick, R. E. Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch. Dermatol. 132(4):395–402, 1996.
Flandin, F., C. Buffevant, and D. Herbage. A differential scanning calorimetry analysis of the age-related changes in the thermal stability of rat skin collagen. Biochim. Biophys. Acta 791:205–211, 1984.
Flory, P. J., and R. R. Garrett. Phase transitions in Collagen and Gelatin systems1. J. Am. Chem. Soc. 80:4836–4845, 1958.
Franchi, M., M. Fini, M. Quaranta, V. De Pasquale, M. Raspanti, G. Giavaresi, et al. Crimp morphology in relaxed and stretched rat Achilles tendon. J. Anat. 210:1–7, 2007.
Hansen, K. A., J. A. Weiss, and J. K. Barton. Recruitment of tendon crimp with applied tensile strain. J. Biomech. Eng. 124:72–77, 2002.
Hayashi, K., et al. The effect of thermal heating on the length and histologic properties of the glenohumeral joint capsule. Am. J. Sports Med. 25(1):107–112, 1997.
Jackson, M., D. S. Johnston, and D. Chapman. Differential scanning calorimetric and fourier transform infrared spectroscopic investigations of cerebroside polymorphism. Biochim. Biophys. Acta 944:497–506, 1988.
Lewandowska, K., A. Sionkowska, S. Grabska, and B. Kaczmarek. Surface and thermal properties of collagen/hyaluronic acid blends containing chitosan. Int. J. Biol. Macromol. 92:371–376, 2016.
Liu, D., M. Nikoo, G. Boran, P. Zhou, and J. M. Regenstein. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 6:527–557, 2015.
Lodish, H., A. Berk, S. L. Zipursky, P. Matsudaira, D. Baltimore, and J. Darnell. Molecular Cell Biology (4th ed.). New York: W. H. Freeman, 2000.
Magnusson, S. P., K. Qvortrup, J. O. Larsen, S. Rosager, P. Hanson, P. Aagaard, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. J. Int. Soc. Matrix Biol. 21:369–377, 2002.
Mellal, I., A. Oukaira, E. Kengene, and A. Lakhssassi. Thermal therapy modalities for cancer treatment: a review and future perspectives. Int. J. Appl. Sci. 4:14, 2017.
Niven, H., E. Baer, and A. Hiltner. Organization of collagen fibers in rat tail tendon at the optical microscope level. Coll. Relat. Res. 2:131–142, 1982.
Pfefer, T.J., B. Choi, G. Vargas, K.M. McNally-Heintzelman, and A.J. Welch. Mechanisms of laser-induced thermal coagulation of whole blood in vitro. In: Lasers in Surgery: Advanced Characterization, Therapeutics, and Systems IX, 1999.
Qin, Z., S. K. Balasubramanian, W. F. Wolkers, J. A. Pearce, and J. C. Bischof. Correlated parameter fit of arrhenius model for thermal denaturation of proteins and cells. Ann. Biomed. Eng. 42:2392–2404, 2014.
Raspanti, M., A. Manelli, M. Franchi, and A. Ruggeri. The 3D structure of crimps in the rat Achilles tendon. Matrix Biol. J. Int. Soc. Matrix Biol. 24:503–507, 2005.
Sun, X.-X., J. Fan, Y.-N. Hou, S. Liang, Y.-P. Zhang, and J.-X. Xiao. Fluorescence characterization of the thermal stability of collagen mimic peptides. Chin. Chem. Lett. 28:963–967, 2017.
Sun, Y., et al. Investigating mechanisms of collagen thermal denaturation by high resolution second-harmonic generation imaging. Biophys. J. 91(7):2620–2625, 2006.
Tanaka, R., et al. In vivo visualization of dermal collagen fiber in skin burn by collagen-sensitive second-harmonic-generation microscopy. J. Biomed. Opt. 18(6):061231, 2013.
Acknowledgments
We thank Enes Atas (scientist in Biomedical Engineering Department at Istanbul Medeniyet University) for helping us to prepare bone samples and Ekin Opar (research assistant in Biomedical Engineering Program at Istanbul Technical University) for analysis and discussing the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
İrem Deniz Derman, Esat Can Şenel, Onur Ferhanoğlu, İnci Çilesiz, and Murat Kazanci declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article and no animal studies were carried out by the authors for this article.
Additional information
Associate Editor Stephanie Michelle Willerth oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Derman, İ.D., Şenel, E.C., Ferhanoğlu, O. et al. Effect of Heat Level and Expose Time on Denaturation of Collagen Tissues. Cel. Mol. Bioeng. 14, 113–119 (2021). https://doi.org/10.1007/s12195-020-00653-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-020-00653-w